Berikut merupakan kutipan ilmiah Kimia Lingkungan yang bermanfaat untuk diketahui sehingga disusun dan digunakan sebagai referensi pribadi.
Perpustakaan Keluarga : Helmut Todo Tua Simamora dan dr. Olga Y.V. Hutapea
In acute cyanide poisoning, cyanide ions (CN–) bind to, and inhibit, the ferric (Fe3+) heme moeity form of mitochondrial cytochrome c oxidase (synonyms: aa3, complex IV, cytochrome A3, EC 1.9.3.1). This blocks the fourth step in the mitochondrial electron transport chain (reduction of O2 to H2O), resulting in the arrest of aerobic metabolism and death from histotoxic anoxia. Tissues that heavily depend on aerobic metabolism such as the heart and brain are particularly susceptible to these effects. Cyanide also binds to other heme-containing enzymes, such as members of the cytochrome p450 family, and to myoglobin. However, these tissue cyanide "sinks" do not provide sufficient protection from histotoxic anoxia. The acute lethal dosage of hydrogen cyanide (HCN) in most animal species is ~2 mg/kg. Plant materials containing ≥200 ppm of cyanogenic glycosides are dangerous.
There are at least two forms of chronic cyanide poisoning in domestic animals: 1) hypothyroidism due to disruption of iodide uptake by the follicular thyroid cell sodium-iodide symporter by thiocyanate, a metabolite in the detoxification of cyanide, and 2) chronic cyanide and plant cyanide metabolite (eg, various glutamyl β-cyanoalanines) -associated neuropathy toxidromes (eg, equine sorghum cystitis ataxia syndrome, cystitis ataxia syndromes in cattle, sheep, and goats).
Etiology
Various chemical forms of cyanides are found in plants, fumigants, soil sterilizers, fertilizers (eg, cyanamide), pesticides/rodenticides (eg, calcium cyanomide) and salts used in industrial processes, such as gold mining, metal cleaning and electroplating, photographic processes, and others. Hydrogen cyanide is also known as prussic acid, and cyanide salts liberate cyanide gas in the presence of acids (eg, in the stomach). Cyanide preparations are still used as vertebrate pest control agents for control of feral pigs, fox, Australian brush-tailed possums, and other pest or predator species in a number of countries. Cyanide salts are still used as killing agents in entomology and (illegally) as a method of fishing and/or collection of aquarium fish species (ie, cyanide fishing). Combustion of common polyacrylonitriles (plastics), wool, silk, keratin, polyurethane (insulation/upholstery), melamine resins (household goods), and synthetic rubber results in the release of cyanide gas. Car fires are notorious sources of cyanide exposure, and cyanide is also a notable component of internal combustion engine exhaust and tobacco smoke. Carbon monoxide poisoning with cyanide gas is thus an extremely common component of smoke inhalation toxidromes.
Toxicity can result from accidental, improper, or malicious use or exposure. However, in livestock species, the most frequent cause of acute and chronic cyanide poisoning is ingestion of plants that either constitutively contain cyanogenic glycosides or are induced to produce cyanogenic glycosides and cyanolipids as a protective response to environmental conditions (plant cyanogenesis). Plant cyanogenesis is a common process and has been documented in >3,000 different plant species distributed over ~110 different families of ferns, gymnosperms, and angiosperms. Of these plants, ~300 species are potential causes of acute and chronic cyanogenic glycoside poisoning, and there are ~75 different cyanogenic glycosides (all of which are O-β-glycosidic derivatives of α-hydroxynitriles). Plant species of notable veterinary importance include Sorghum spp (Johnson grass, Sudan grass, and S bicolor, the common cereal grain crop referred to as "sorghum" or the synonyms durra, jowari, milo),Acacia greggii (guajillo), Amelanchier alnifolia (western service berry), Linum spp (linseeds and flaxes),Sambucus nigra (elderberry), Suckley suckleyana (poison suckleya), Triglochin maritima and T palustris(marsh arrow grasses), Mannihot esculentum (cassava), all members of the Prunus genus until proved otherwise (apricot, peach, chokecherry, pincherry, wild black cherry, ornamental cherry, peaches, nectarines, apricots, almonds, bird cherries, black thorn, cherry laurels [commercial orchard species are often specifically bred for low cyanide content; however, ornamental members of this genus are often highly poisonous]), Nandina domestica (heavenly or sacred bamboo), Phaseolus lunatus (Lima beans), members of the Vicia genus until proved otherwise (vetches; often pasture species have been bred for low cyanogenesis), Lotus spp (bird's-foot treefoils; often, pasture species have been bred for low cyanogenesis), Trifolium sp (clovers; often, pasture species have been bred for low cyanide content),Zea mays (corn), Eucalyptus spp (gum trees), Hydrangia spp (hydrangias), Pteridium aquillinum (bracken fern), Bahia oppositifolia (bahia), and Chaenomales spp (flowering quince) (Also see Sorghum Poisoning). A number of insect species are also able to synthesize hydrogen cyanide and/or sequester hydrogen cyanide that is derived from the cyanogenic glycosides of their plant hosts (notably the USA eastern tent caterpillar Malacosoma americanum that is associated with mare reproductive loss syndrome (also seeMare Reproductive Loss Syndrome); however, cyanide is not the cause of mare reproductive loss syndrome. Invertebrates such as Burnet moths (Zygaena spp) that feed on bird's-foot trefoils), as well as certain centipede and millipedes, are potentially hazardous food sources for exotic pet species.
Plant cyanogenesis in response to environmental stressors is an important part of the etiology and risk of acute cyanogenic glycoside poisoning. Within plants, amino acids that are not used for protein synthesis can be metabolized to α-hydroxynitriles and then to cyanogenic glycosides. Plants are protected from the potential adverse effects of cyanogenic glycosides by two features: cyanogenic glycosides are largely found within cell vacuoles, and the presence of the detoxifying enzyme β-cyanoalanine synthase (which is responsible for production of some of the cyanide derivatives putatively involved in the chronic cyanide-associated neurologic toxidromes). Even so-called "acyanogenic" plants can become toxic under appropriate environmental circumstances. Environmental conditions that damage relevant plant species, reduce protein synthesis, enhance the conversion of nitrate to amino acids in the presence of reduced protein synthesis, and/or inhibit β-cyanoalanine synthase potentially increase the risk of cyanogenesis. Relevant environmental factors include crushing, wilting, freezing, high environmental temperatures, herbicide treatment, water stress, cool moist growing conditions, nitrate fertilization, high soil nitrogen:phosphorus ratios, soil phosphorus deficiency, low soil sulfur (decreases detoxification of cyanogenic glycosides to thiocyanates within plants), insect attack, and various plant diseases. Herbicide treatment of plants is important in that it may also increase plant palatability. Crushing and/or mastication of potentially cyanogenic plants is important in development of the acute toxidrome, because this releases cyanogenic glycosides from plant cell vacuoles and exposes them to catabolism by β-glucosidase and hydroxynitrile lyase present in the plant cell cytosol. Young, rapidly growing areas of plants and areas of regrowth after cutting often have high cyanogenic glycoside content. As a rough approximation, rapidly growing Sorghum spp are often hazardous until they reach ~60 cm in height; however, this is no guarantee of safety, and if there is any doubt regarding cyanogenic potential, samples of potential forage should be tested. Plant seeds and leaves typically have higher cyanogenic potential, while the fleshy parts of fruits generally have low levels. Drying often increases the cyanogenic potential of plants, whereas ensiling may reduce cyanide content by ~50%.
β-glucosidase and hydroxynitrile lyase are also present in the rumen microflora, and a rumen pH of ~6.5–7 favors conversion of cyanogenic glycosides to cyanide. Ruminants on high-energy grain rations are somewhat less susceptible, because their lower rumen pH (~4–6) reduces the formation of cyanide. Consumption of water before grazing on cyanogenic pastures appears to increase the risk. Monogastric animals with low stomach pH are also somewhat less susceptible to cyanogenic glycoside poisoning. However, these factors do not guarantee immunity from poisoning.
Under conditions of low-level exposure, mammals detoxify ~80% of ingested cyanide to thiocyanate via mitochondrial rhodanese. Thiocyanate is then largely excreted in urine. Often, the rate of the rhodanese pathway is limited by the availability of thiosulfate; also notably, dogs have lower overall rhodanese activity than other species. Minor, but toxicologically important, pathways of detoxification in mammals include the combination of cyanide with hydroxycobalamin (vitamin B12a) to yield cyanocobalamin (vitamin B12), and the nonenzymatic combination of cyanide with cysteine to form β-thiocyanoalanine, which is converted to 2-iminothiazolidine-4-carboxylic acid and subsequently excreted. Small amounts of β-thiocyanoalanine are also excreted in saliva. Dietary levels of sulfur amino acids (L-cysteine and L-methionine) strongly influence the rate of detoxification of cyanide, and low dietary intakes are associated with higher blood cyanide levels, particularly under conditions of chronic, low level exposure. Dietary sulfur and sulfur amino acid intake are known to strongly affect the neurologic toxidromes associated with chronic cyanide/cyanogenic glycoside exposure in people.
Chronic low-level cyanide/cyanogenic glycoside exposure is associated with increased exposure to the cyanide metabolite thiocyanate. Under conditions of thiocyanate overload, thiocyanate acts as a competitive inhibitor of thyroid follicular cell iodine uptake by the sodium/iodide symporter. This results in reduced iodination of tyrosine, reduced T3 synthesis, increased blood TSH, goiter, and hypothyroidism. Similar effects occur with some plant glucosinolates (goitrogenic glycosides). Selenium deficiency appears to enhance these effects.
Chronic, low-level cyanide/cyanogenic glycoside exposure (often in combination with low dietary sulfur and/or sulfur amino acid intake) is associated with neuropathy syndromes in horses and ruminants. Sorghum cystitis ataxia syndrome of horses is associated with diffuse nerve fiber degeneration in the lateral and ventral funiculi of the spinal cord and brain stem. Similar syndromes have been described in ruminants. Comparisons between these syndromes as chronic cyanogenic glycoside–associated human myeloneuropathies such as Konzo and tropical ataxic neuropathy have been made; however, the precise toxins and modes of action are yet to be fully defined. All of these toxidromes appear to be related to a combination of chronic cyanide/cyanogenic glycoside exposure combined with low dietary sulfur and/or sulfur amino acid intake and possibly other nutritional deficiencies. Lathyrogenic plant cyanide metabolites such as β-cyanoalanine have been implicated as causative or at least contributory agents.
Chronic, low-level cyanogenic glycoside exposure (notably from Sorghum spp) has been associated with musculoskeletal teratogenesis (ankyloses or arthrogryposes) and abortion.
Clinical Findings
Acute cyanide poisoning: Signs generally occur within 15–20 min to a few hours after animals consume toxic forage, and survival after onset of clinical signs is rarely >2 hr. Excitement can be displayed initially, accompanied by rapid respiration rate. Dyspnea follows shortly, with tachycardia. The classic "bitter almond" breath smell may be present; however, the ability to detect this smell is genetically determined in people, and anosmic people (a significant proportion of the population) cannot detect it. Salivation, excess lacrimation, and voiding of urine and feces may occur. Vomiting may occur, especially in pigs. Muscle fasciculation is common and progresses to generalized spasms and coma before death. Animals may stagger and struggle before collapse. In other cases, sudden unexpected death may ensue. Mucous membranes are bright red but may become cyanotic terminally. Venous blood is classically described as "cherry red" because of the presence of high venous blood pO2; however, this color rapidly changes after death. Serum ammonia and neutral and aromatic amino acids are typically increased. Cardiac arrhythmias are common due to myocardial histotoxic hypoxia. Death occurs during severe asphyxial convulsions. The heart may continue to beat for several minutes after struggling, and breathing stops. The elimination half-life of cyanide in dogs is reported to be 19 hr, so prognosis of recovery without therapeutic intervention is grave: it would take more than 4 days to eliminate >95% of the cyanide present.
Chronic cyanide poisoning syndromes: Chronic cyanogenic glycoside hypothyroidism will present as hypothyroidism with or without goiter. Cystitis ataxia toxidromes are typically associated with posterior ataxia or incoordination that may progress to irreversible flaccid paralysis, cystitis secondary to urinary incontinence, and hindlimb urine scalding and alopecia. Death, although uncommon, is often associated with pyelonephritis. Late-term abortion and musculoskeletal teratogenesis may also occur.
Lesions:
Acute cyanide poisoning: Necropsy personnel may require appropriate personal protective equipment, including respirators with suitable cartridges. Venous blood is classically described as being "bright cherry red"; however, this color rapidly fades after death or if the blood is exposed to the atmosphere. Whole blood clotting may be slow or not occur. Mucous membranes may also be pink initially, then become cyanotic after respiration ceases. The rumen may be distended with gas; in some cases the odor of “bitter almonds” may be detected after opening. Rumen contents may provide a positive sodium picrate paper test (or positive results on other rapid cyanide test strip systems). Rumen gases may provide positive results in cyanide Draeger tube rapid test systems. Agonal hemorrhages of the heart may be seen. Liver, serosal surfaces, tracheal mucosa, and lungs may be congested or hemorrhagic; some froth may be seen in respiratory passages. Cyanide also binds to iron (both Fe2+ and Fe3+) present in myoglobin (although this occurs more slowly than the binding to cytochrome c oxidase and, hence, is not protective); this may result in a generalized dark coloration of skeletal muscle. Neither gross nor histologic lesions are consistently seen.
Multiple foci of degeneration or necrosis may be seen in the CNS of dogs chronically exposed to sublethal amounts of cyanide. These lesions have not been reported in livestock.
Chronic cyanide poisoning: Goiter may be present. Cystitis ataxia toxidromes are characterized by opportunistic bacterial cystitis with or without pyelonephritis and diffuse nerve fiber degeneration in the lateral and ventral funiculi of the spinal cord and brain stem. Hindlimb urine scalding and alopecia may be present.
Diagnosis
Appropriate history, clinical signs, postmortem findings, and demonstration of HCN in rumen (stomach) contents or other diagnostic specimens support a diagnosis of cyanide poisoning. Veterinarians should be aware of the possible need to use appropriate personal protective equipment, including a respirator, when collecting samples that may liberate cyanide gas (eg, rumen contents and rumen gas cap). A rapid qualitative and presumptive diagnosis can be made by testing representative plant samples or stomach contents using the picric acid paper test or by collecting rumen gas cap samples by trocarization and testing with a Draeger cyanide gas detection tube or other cyanide gas detection system. Negative results with such rapid presumptive tests do not completely exclude the possibility of cyanide poisoning. Suitable specimens for more sophisticated testing include the suspected food source, rumen/stomach contents, samples of the rumen gas cap, heparinized whole blood, liver, and muscle. Antemortem whole blood is preferred; other specimens should be collected as soon as possible after death, preferably within 4 hr. Specimens should be sealed in an airtight container, refrigerated or frozen, and submitted to the laboratory without delay. When cold storage is unavailable, immersion of specimens in 1%–3% mercuric chloride has been satisfactory. The rationale for using liver as a diagnostic sample is that cyanide binds to the Fe3+ form of cytochrome p450 and other heme-containing metabolic enzymes. The rationale for using skeletal muscle is that cyanide will bind to the iron moiety in myoglobin.
Where available, measurement of the urinary metabolite of cyanide, thiocyanate, may reveal increased concentrations after cyanide poisoning.
Hay, green chop, silage, or growing plants containing >220 ppm cyanide as HCN on a wet-weight (as is) basis are very dangerous as animal feed. Forage containing <100 ppm HCN, wet weight, is usually safe to pasture. Analyses performed on a dry-weight basis have the following criteria: >750 ppm HCN is hazardous, 500–750 ppm HCN is doubtful, and <500 ppm HCN is considered safe.
Normally expected cyanide concentrations in blood of most animal species are usually <0.5 mcg/mL. Minimal lethal blood concentrations are ~3 mcg/mL or less. Cyanide concentrations in muscle are similar to those in blood, but concentrations in liver are generally lower than those in blood. In dogs, whole blood cyanide concentrations may be 4–5 times greater than serum concentrations because of binding to ferric ions and sequestration in RBCs.
Differential diagnoses include poisonings by nitrate or nitrite, urea, organophosphate, carbamate, chlorinated hydrocarbon pesticides, and toxic gases (carbon monoxide and hydrogen sulfide), as well as infectious or noninfectious diseases and other toxidromes that cause sudden death.
Treatment, Control, and Prevention
Immediate treatment is necessary. The goal of treatment is to break the cyanide-cytochrome c oxidase bond and reestablish the mitochondrial electron transport chain. One way to accomplish this is by using inducing Fe3+ in hemoglobin (ie, inducing methemoglobinemia), which then acts as a high-affinity decoy chemical receptor for cyanide and forms cyanmethemoglobin. Classically, various nitrites have been used for this purpose; eg, inhaled amyl nitrite followed by IV injection of a nitrite salt (typically sodium nitrite) has been used to rapidly induce methemoglobinemia. Cyanide bound to methemoglobin can then be detoxified by rhodanese to thiocyanate. Because the rhodanese-mediated detoxification of cyanide to thiocyanate is usually capacity and rate limited by the availability of sulfur donors, treatment with nitrites is usually followed up by injection of sodium thiosulfate. Oral dosing with sodium thiosulfate into the rumen and/or stomach has also been suggested because the reaction between thiosulfate and cyanide can also occur nonenzymatically, and this may reduce any ongoing production of cyanide in the rumen/stomach environments.
If possible, the contents of one 0.3-mL vial of amyl nitrite should be inhaled by the animal as soon as possible after exposure, followed by an IV infusion of sodium nitrite (10 g/100 mL of distilled water or isotonic saline; 20 mg/kg body wt) over 3-4 min. Nitrite treatment is then followed by a slow IV injection of sodium thiosulfate (20% w/w) at ≥500 mg/kg. Thiosulfate is generally well tolerated; however, vomiting and hypotension can occur. The thiosulfate injection can be repeated if necessary. Oral administration of thiosulfate can also be considered in an attempt to convert any cyanide in the stomach/rumen into thiocyanate. Sodium nitrite therapy may be carefully repeated at 10 mg/kg, every 2–4 hr or as needed. Ideally, decisions regarding repeated treatment with nitrites should consider the degree of methemoglobinemia present.
Notably, thiosulfate treatment alone has been successful in some cases. However, thiosulfate treatment should ideally be preceded by nitrite induction of methemoglobinemia in cases of confirmed cyanide poisoning. However, because thiosulfate is generally well tolerated, it is often administered alone in situations when cyanide exposure is likely but unconfirmed (eg, smoke inhalation or exposure to fires).
Hydroxocobalamin (vitamin B12a ) is also used as a cyanide antidote. Hydroxocobalamin detoxifies cyanide by binding to it and forming cyanocobalamin (ie, another decoy receptor approach), which is then excreted in urine. It has the advantages that it is relatively well tolerated, does not compromise blood oxygen-carrying capacity, and does not produce hypotension. Hydroxocobalamin does produce chromaturia (which may result in false urinalysis results), as well as infusion site reactions, GI upset, pruritus, and dysphagia. The suggested dosage is 70 mg/kg, infused IV over 15 min, repeated as necessary.
Sulfanegen (as the sodium or triethanolamine salt) has been developed for treatment of cyanide mass poisoning incidents. This approach has the advantage that sulfanegen is water soluble and can be administered IM. Sulfanegen is a prodrug that generates 3-mercaptopyruvic acid (3-MP), an intermediate in cysteine metabolism, which again acts as a decoy receptor for cyanide. By itself, the half-life of 3-MP is too short to be effective against cyanide poisoning. For this reason, prodrugs such as sulfanegen have been developed to increase the duration of action of 3-MP in vivo.
Alternative inducers of methemoglobinemia such as 4-dimethyl-aminophenol (DMAP; IM at 5 mg/kg) or hydroxylamine hydrochlorine (IM at 50 mg/kg) have been suggested, because they produce methemoglobinemia more quickly than the nitrites currently in use. However, these hemoglobin-oxidizing agents are also relatively toxic to RBCs and can induce severe effects such as hemolysis and renal damage. These "rapid agents" still have the disadvantage of reducing blood oxygen-carrying capacity.
Other alternative antidotes in clinical development and use worldwide include dicobalt-ethylenediaminetetraacetic acid (EDTA) and α−ketoglutaric acid. Although hydroxycobalamin has been approved by the FDA for use in the USA, none of the others is readily available. Dicobalt-EDTA releases cobalt ions that react with cyanide ions; highly stable cyanide-cobalt complexes are then excreted by the kidneys. This drug is very potent and has immediate action but is reported to have numerous, severe adverse effects in people. The investigational antidote α-ketoglutaric acid has a molecular configuration that renders it amenable to nucleophilic binding of cyanide without generation of methemoglobin. Pretreatment with this drug reduced lethal outcomes and increased efficacy of sodium thiosulfate, but postexposure efficacy in animals is unknown.
Sodium thiosulfate alone is also an effective antidotal therapy at ≥500 mg/kg, IV, plus 30 g/cow, PO, to detoxify any remaining HCN in the rumen. When available, oxygen should be used to supplement nitrite or thiosulfate therapy, especially in small animals. Hyperbaric oxygen therapy (100% oxygen breathed intermittently at a pressure >1 atmosphere absolute) causes an above-normal partial pressure of oxygen (PO2) in arterial blood and markedly increases the amount of oxygen dissolved in plasma. Oxygen-dependent cellular metabolic processes benefit from heightened oxygen tension in capillaries and enhanced oxygen diffusion from capillaries to critical tissues. Activated charcoal does not effectively absorb cyanide and thus is not recommended PO for antidotal therapy.
Caution is indicated in treatment. All cyanide antidotes are toxic by themselves. Many clinical signs of nitrate and prussic acid poisoning are similar, and injecting sodium nitrite induces methemoglobinemia identical to that produced by nitrite poisoning. If in doubt of the diagnosis, methylene blue, IV, at 4–22 mg/kg, may be used to induce methemoglobin. Because methylene blue can serve as both a donor and acceptor of electrons, it can reduce methemoglobin in the presence of excess methemoglobin or induce methemoglobin when only hemoglobin is present (but sodium nitrate is the more effective treatment for cyanide poisoning if the diagnosis is certain).
The best preventive step is to test suspect feed and/or pastures before allowing consumption. Pasture and forage sorghums (eg, Sudan grass and sorghum-Sudan grass hybrids) should not be grazed until they are >60 cm tall or have been proved by testing to have acceptable cyanide levels, to reduce danger from prussic acid poisoning. Animals should be fed before first turning out to pasture; hungry animals may consume forage too rapidly to detoxify HCN released in the rumen. Animals should be turned out to new pasture later in the day; potential for prussic acid release is reported to be highest during early morning hours. Free-choice salt and mineral with added sulfur may help protect against prussic acid toxicity. Grazing should be monitored closely during periods of environmental stress, eg, drought or frost. Abundant regrowth of sorghum can be dangerous; these shoots should be frozen and wilted before grazing.
Green chop forces livestock to eat both stems and leaves, thereby reducing problems caused by selective grazing. Cutting height can be raised to minimize inclusion of regrowth.
Sorghum hay and silage usually lose ≥50% of prussic acid content during curing and ensiling processes. Free cyanide is released by enzyme activity and escapes as a gas. Although a rare occurrence, hazardous concentrations of prussic acid may still remain in the final product, especially if the forage had an extremely high cyanide content before cutting. Hay has been dried at oven temperatures for up to 4 days with no significant loss of cyanide potential. These feeds should be analyzed before use whenever high prussic acid concentrations are suspected. Potentially toxic feed should be diluted or mixed with grain or forage that is low in prussic acid content to achieve safe concentrations in the final product. At least in theory, the risk of chronic cyanide poisoning syndromes may be reduced by iodine supplementation in the case of hypothyroidism and by sulfur-containing amino acids in the case of chronic neurologic toxidromes. Great care must be taken when providing supplemental elemental sulfur sources in ruminants because of the possible risk of polioencephalomalacia (see Polioencephalomalacia).
Cyanide poisoning occurs when a living organism is exposed to a compound that produces cyanide ions (CN−) when dissolved in water. Common poisonous cyanide compounds include hydrogen cyanide gas and the crystalline solidspotassium cyanide and sodium cyanide. The cyanide ion halts cellular respiration by inhibiting an enzyme in the mitochondria called cytochrome c oxidase.
| Cyanide poisoning | |
|---|---|

Cyanide ion
| |
| Classification and external resources | |
| Specialty | Toxicology, critical care medicine |
| ICD-10 | T65.0 |
| ICD-9-CM | 989.0 |
| DiseasesDB | 3280 |
| eMedicine | med/487 |
Cyanide poisoning is a form of histotoxic hypoxia because the cells of an organism are unable to use oxygen, primarily through the inhibition of cytochrome c oxidase. Acute hydrogen cyanide poisoning can result from inhalation of fumes from burning polymer products that use nitrile in their production, such as polyurethane, or vinyl.[1] It can also be caused by breakdown of nitroprusside into nitric oxide and cyanide during treatment of hypertensive crises. If cyanide is inhaled it causes a coma with seizures, apnea, and cardiac arrest, with death following in a matter of seconds. At lower doses, loss of consciousness may be preceded by general weakness, giddiness, headaches, vertigo, confusion, and perceived difficulty in breathing. At the first stages of unconsciousness, breathing is often sufficient or even rapid, although the state of the victim progresses towards a deep coma, sometimes accompanied by pulmonary edema, and finally cardiac arrest. A cherry red skin color that changes to dark may be present as the result of increased venous hemoglobin oxygen saturation. Cyanide does not directly cause cyanosis. A fatal dose for humans can be as low as 1.5 mg/kg body weight.
In addition to its uses as a pesticide and insecticide, cyanide is contained in tobacco smoke and smoke from building fires, and is present in some foods such asalmonds, apricot kernel, apple seeds, orange seeds, cassava (also known as yuca or manioc), and bamboo shoots. Vitamin B12, in the form of hydroxocobalamin(also spelled hydroxycobalamin), may reduce the negative effects of chronic exposure, and a deficiency can lead to negative health effects following exposure.[3]
Exposure to lower levels of cyanide over a long period (e.g., after use of improperly processed cassava roots as a primary food source in tropical Africa) results in increased blood cyanide levels, which can result in weakness and a variety of symptoms, including permanent paralysis, nervous lesions,[4][5][6] hypothyroidism,[5]and miscarriages.[7][8] Other effects include mild liver and kidney damage.[9][10]
Cyanide has the potential to be bioremediated. Among the enzyme families which degrade cyanide are nitrogenases, rhodanese, and the nitrilases. Cyanide hydratases, part of the nitrilase enzymes that convert cyanide are present in certain species of fungi hydrolyze cyanide to formamide, Cyanide dihydratases convert it directly to formate and ammonia, end products that pose far less toxicity.[11]
The United States standard cyanide antidote kit first uses a small inhaled dose of amyl nitrite, followed by intravenous sodium nitrite, followed by intravenous sodium thiosulfate.[12] Hydroxocobalamin is newly approved in the US and is available in Cyanokit antidote kits.[13] Sulfanegen TEA, which could be delivered to the body through an intra-muscular (IM) injection, detoxifies cyanide and converts the cyanide into thiocyanate, a less toxic substance.[14] Alternative methods of treating cyanide intoxication are used in other countries.
| Agent | Description |
|---|---|
| Nitrites | The nitrites oxidize some of the hemoglobin's iron from the ferrous state to the ferric state, converting the hemoglobin into methemoglobin.
Cyanide binds avidly to methemoglobin, forming cyanmethemoglobin, thus releasing cyanide from cytochrome oxidase.[15] Treatment withnitrites is not innocuous as methemoglobin cannot carry oxygen, and methemoglobinemia needs to be treated in turn with methylene blue.
|
| Thiosulfate | The evidence for sodium thiosulfate's use is based on animal studies and case reports: the small quantities of cyanide present in dietary sources and in cigarette smoke are normally metabolized to relatively harmless thiocyanate by the mitochondrial enzyme rhodanese(thiosulfate cyanide sulfurtransferase), which uses thiosulfate as a substrate. However, this reaction occurs too slowly in the body for thiosulfate to be adequate by itself in acute cyanide poisoning. Thiosulfate must therefore be used in combination with nitrites.[15] |
| Hydroxocobalamin | Hydroxocobalamin, a form (or vitamer) of vitamin B12 made by bacteria, and sometimes denoted vitamin B12a, is used to bind cyanide to form the harmless cyanocobalamin form of vitamin B12. |
| 4-Dimethylaminophenol | 4-Dimethylaminophenol (4-DMAP) has been proposed[by whom?] in Germany as a more rapid antidote than nitrites with (reportedly) lower toxicity. 4-DMAP is used currently by the German military and by the civilian population. In humans, intravenous injection of 3 mg/kg of 4-DMAP produces 35 percent methemoglobin levels within 1 minute. Reportedly, 4-DMAP is part of the US Cyanokit, while it is not part of the German Cyanokit due to side effects (e. g. hemolysis). |
| Dicobalt edetate | Cobalt ions, being chemically similar to iron ions, can also bind cyanide. One current cobalt-based antidote available in Europe is dicobalt edetate or dicobalt-EDTA, sold as Kelocyanor. This agent chelates cyanide as the cobalticyanide. This drug provides an antidote effect more quickly than formation of methemoglobin, but a clear superiority to methemoglobin formation has not been demonstrated. Cobaltcomplexes are quite toxic, and there have been accidents reported in the UK where patients have been given dicobalt-EDTA by mistake based on a false diagnosis of cyanide poisoning. Because of its side effects, it should be reserved only for patients with the most severe degree of exposure to cyanide; otherwise, nitrite/thiosulfate is preferred.[16] |
| Glucose | Evidence from animal experiments suggests that coadministration of glucose protects against cobalt toxicity associated with the antidote agent dicobalt edetate. For this reason, glucose is often administered alongside this agent (e.g. in the formulation 'Kelocyanor'). It has also been anecdotally suggested that glucose is itself an effective counteragent to cyanide, reacting with it to form less toxic compounds that can be eliminated by the body. One theory on the apparent immunity of Grigory Rasputin to cyanide was that his killers put the poison in sweet pastries and madeira wine, both of which are rich in sugar; thus, Rasputin would have been administered the poison together with massive quantities of antidote. One study found a reduction in cyanide toxicity in mice when the cyanide was first mixed with glucose.[17] However, as yet glucose on its own is not an officially acknowledged antidote to cyanide poisoning. |
| 3-Mercaptopyruvateprodrugs | The most widely studied cyanide-metabolizing pathway involves utilization of thiosulfate by the enzyme rhodanese, as stated above. In humans, however, rhodanese is concentrated in the kidneys (0.96 units/mg protein) and liver (0.15 u/mg), with concentrations in lung, brain, muscle and stomach not exceeding 0.03 U/ml.[18] In all these tissues, it is found in the mitochondrial matrix, a site of low accessibility for ionized, inorganic species, such as thiosulfate. This compartmentalization of rhodanese in mammalian tissues leaves major targets of cyanide lethality, namely, the heart and central nervous system, unprotected. (Rhodanese is also found in red blood cells, but its relative importance has not been clarified.[19][20])
A different cyanide-metabolizing pathway, 3-mercaptopyruvate sulfurtransferase (3-MPST, EC 2.8.1.2), which is more widely distributed in mammalian tissues than rhodanese, is being explored. 3-MPST converts cyanide to thiocyanate, using the cysteine catabolite, 3-mercaptopyruvate (3-MP). However, 3-MP is extremely unstable chemically. Therefore, a prodrug, sulfanegen sodium (2, 5-dihydroxy-1,4-dithiane-2,5-dicarboxylic acid disodium salt), which hydrolyzes into 2 molecules of 3-MP after being administered orally or parenterally, is being evaluated in animal models.[21][22]
|
| Oxygen therapy | Oxygen therapy is not a cure in its own right. However, the human liver is capable of metabolizing cyanide quickly in low doses (smokers breathe in hydrogen cyanide, but it is such a small amount and metabolized so fast that it does not accumulate).
The International Programme on Chemical Safety issued a survey (IPCS/CEC Evaluation of Antidotes Series) that lists the following antidotal agents and their effects: oxygen, sodium thiosulfate, amyl nitrite, sodium nitrite, 4-dimethylaminophenol, hydroxocobalamin, and dicobalt edetate ('Kelocyanor'), as well as several others.[23] Other commonly-recommended antidotes are 'solutions A and B' (a solution offerrous sulfate in aqueous citric acid, and aqueous sodium carbonate, respectively) and amyl nitrite.
The UK Health and Safety Executive (HSE) has recommended against the use of solutions A and B because of their limited shelf life, potential to cause iron poisoning, and limited applicability (effective only in cases of cyanide ingestion, whereas the main modes of poisoning are inhalation and skin contact). The HSE has also questioned the usefulness of amyl nitrite due to storage/availability problems, risk of abuse, and lack of evidence of significant benefits. It also states that the availability of Kelocyanor at the workplace may mislead doctors into treating a patient for cyanide poisoning when this is an erroneous diagnosis. The HSE no longer recommends a particular cyanide antidote.[24] Qualified UK first aiders are now only permitted to apply oxygen therapy using a bag valve mask, providing they have been trained in its usage.
|
History
Burnings
- On December 5, 2009, a fire in the night club Lame Horse (Khromaya Loshad) in the Russian city of Perm took the lives of 156 people. 111 people died on the spot and 45 later in hospitals. One of the main causes of death was poisoning from cyanide and other toxic gases released by the burning of plastic andpolystyrene foam used in the construction of club interiors. Taking into account the number of deaths, this was the largest fire in post-Soviet Russia.[citation needed]
- On January 27, 2013, a fire at the Kiss nightclub in the city of Santa Maria, in the south of Brazil, caused the poisoning of hundreds of young people by cyanide released by the combustion of soundproofing foam made with polyurethane. By March 2013, 241 fatalities were confirmed.[25][26]
Gas chambers
- Hydrogen cyanide in the form of Zyklon B was used in German extermination camps during World War II, and especially from March 1942 onwards, when it was first used experimentally to murder Russian prisoners of war at Auschwitz. Use of the poison was scaled up rapidly until custom-built gas chambers (holding up to about 2000 victims) were constructed as part of the new crematoria complex at Auschwitz-Birkenau. There was also a large undressing room next to the gas chamber, and the victims were told to undress and leave their clothes on a numbered peg for collection later. They were told that they would receive a hot shower, and false shower heads were fitted in the ceilings of the gas chambers, so as to maintain the deception. The gas chambers were sealed hermetically to prevent gas leakage. The Zyklon B pellets were then dropped into the chamber via small openings in the roof. When the pellets were exposed to moisture and human heat (as in a closed chamber), they gave off gaseous HCN, which then killed the victims. Workers in theSonderkommando were employed to remove the corpses from the gas chamber and strip them of any valuables, such as gold teeth, before the bodies were cremated. The gas was used mainly at Auschwitz and Majdanek, but theextermination camps such as Treblinka built earlier used engine exhaust gas, in which carbon monoxide was the toxic component. The gas chambers were either mobile lorries as at Chelmno or specially built chambers as at Sobibor andBelzec. The victims included prisoners of war, Jews from across Europe, Romani gypsies, Poles, ill and disabled peopleof all nationalities, as well as political prisoners, homosexuals, Jehovah's witnesses and anyone who opposed the Nazis.
- Hydrogen cyanide gas has also been used for judicial execution in some states of the United States, where cyanide was generated by reaction between potassium cyanide (or sodium cyanide[27][28]) dropped into a compartment containingsulfuric acid, directly below the chair in the gas chamber.[29] The State of California executed Caryl Chessman in this manner.
War
Cyanide was stockpiled in chemical weapons arsenals in both the Soviet Union and the United States in the 1950s and 1960s.[citation needed] However, as a military agent, hydrogen cyanide was not considered very effective, since it is lighter than air and needs a significant dose to incapacitate or kill.
Suicide
See also: Category:Suicides by cyanide poisoning.
Cyanide salts are sometimes used as fast-acting suicide devices. Cyanide reacts at a higher level with high stomach acidity.
- In February 1937, the Uruguayan short story writer Horacio Quiroga committed suicide by drinking cyanide in a hospital at Buenos Aires.
- In 1937, the famous polymer chemist, Wallace Carothers, committed suicide by cyanide.
- In the 1943 Operation Gunnerside, to destroy the Vemork Heavy Water Plant in World War II (an attempt to stop/slow German atomic bomb progress), the commandos were given cyanide tablets (cyanide enclosed in rubber) kept in the mouth and were instructed to bite into them in case of German capture. The tablets ensured death within three minutes.[30]
- Cyanide, in the form of pure liquid prussic acid (a historical name for hydrogen cyanide), was the favored suicide agent of the Third Reich. It was used to commit suicide by Erwin Rommel (1944), after being accused of conspiring against Hitler; Adolf Hitler's wife, Eva Braun (1945); and by Nazi leaders Heinrich Himmler(1945), possibly Martin Bormann (1945), and Hermann Göring (1946).
- It is speculated that, in 1954, Alan Turing used an apple that had been injected with a solution of cyanide to commit suicide after being convicted of having a homosexual relationship—illegal at the time in the UK—and forced to undergo hormonal castration.
- Jonestown, Guyana, was the site of a large mass suicide/murder, in which over 900 members of the Peoples Temple drank potassium cyanide–laced Flavor Aidin 1978.
- Members of the Sri Lankan LTTE (Liberation Tigers of Tamil Eelam, whose insurgency lasted from 1983 to 2009), used to wear cyanide vials around their necks with the intention of committing suicide if captured by the government forces.
- On June 28, 2012, millionaire Wall Street trader Michael Marin ingested a cyanide pill seconds after a guilty verdict was read in his arson trial in Phoenix, AZ; he died minutes after.[31]
- On June 27, 2013, after being found guilty of statutory sodomy of a 14-year-old female, 48-year-old Steve Parsons ingested a cyanide pill and died shortly after in Maryville, MO.[32][33][34]
Mining and industrial
- In 2000, a spill at Baia Mare, Romania resulted in the worst environmental disaster in Europe since Chernobyl.[35]
- In 2000, Allen Elias,[36] CEO of Evergreen Resources was convicted of knowing endangerment for his role in the cyanide poisoning of employee Scott Dominguez.[37][38] This was one of the first successful criminal prosecutions of a corporate executive by the Environmental Protection Agency.
Murder
See:
- John Tawell murderer, who in 1845 became the first person to be arrested as the result of telecommunications technology
- Grigori Rasputin (1916; attempted, later killed by gunshot)
- Goebbels children (1945)
- Nick Baird and Casey Killen (2013)
- Chicago Tylenol murders (1982)
- Ronald Clark O'Bryan (1944-1984)
- Richard Kuklinski (1948–2006)
- Neil Heywood (1970-2011)[39]
- Dr. Autumn Marie Klein (1972-2013)[40]
- On July 19, 2012, Urooj Khan, 46, cashed in an Illinois lottery ticket for more than $600,000. After taxes, the winnings amounted to about $425,000. Khan fell ill the next day and was pronounced dead at a hospital. No autopsy was done because, at the time, the Chicago Medical Examiner's Office didn't generally perform them on people 45 and older unless the death was suspicious. The cutoff age has since been raised to 50. After the basic toxicology screening for opiates, cocaine and carbon monoxide came back negative, the death was ruled a result of the narrowing and hardening of coronary arteries. Days after the initial cause of death was released, a relative of Khan's asked authorities to look into the case further. The morgue reopened the case and did more extensive toxicology studies. On March 1, 2013, the Cook County coroner's office confirmed Khan was the victim of cyanide poisoning.[41]
Terrorism
- In 1995, a device was discovered in a restroom in the Kayabacho Tokyo subway station, consisting of bags of sodium cyanide and sulfuric acid with a remote controlled motor to rupture them in what was believed to be an attempt by the Aum Shinrikyo cult to produce toxic amounts of hydrogen cyanide gas.[42]
- In 2003, Al Qaeda reportedly planned to release cyanide gas into the New York City Subway system. The attack was supposedly aborted because there would not be enough casualties.[43]
In fiction
Homicide
- The Detective Conan manga/anime series has a large number of cases in which the victims are killed by cyanide, with all or most mentioning an 'almond scent' to describe it.
- Raymond Chandler uses "a little potassium hydrocyanide" against private detective Philip Marlowe in The Little Sister – "merely relaxing".
- In Agatha Christie's And Then There Were None, the first death occurs from cyanide poisoning.
- In Agatha Christie's Sparkling Cyanide (also entitled Remembered Death), based upon her Hercule Poirot short story entitled "Yellow Iris", Rosemary and George Barton are poisoned by cyanide crystals.
- In Ngaio Marsh's Death At The Bar, Luke Watchman, a top London barrister and King's Counsel dies of potassium cyanide poisoning after a freak dart throwing accident whilst holidaying in Devon.
- In Hit Man: A Technical Manual for Independent Contractors by "Rex Feral", the use of cyanide to poison a mark is explained in detail.
- In the Joseph Kesselring play Arsenic and Old Lace, two old ladies mix wine with arsenic, cyanide and strychnine to use to kill old men.
- In Roald Dahl's short story "The Landlady", a landlady poisons a young boy named Billy Weaver staying in her Bed and Breakfast with tea that was said to taste suspiciously like cyanide (described as tasting like "bitter almonds"), presumably to stuff him.
- Bishop Lilliman was killed by 'V', forcing the bishop to swallow a communion wafer poisoned with cyanide in Alan Moore and David Lloyd's comic book series, V for Vendetta.
- In the manga Battle Royale, Yuko Sakaki's assigned weapon is hydrocyanic acid. She uses it to poison food intended for Shuuya Nanahara, but it ends up being ingested by Yuka Nakagawa.
- In Phoenix Wright: Ace Attorney: Trials and Tribulations game, the third case solved by the player involves a programmer who is murdered when potassium cyanide is slipped into his coffee at a restaurant.
- In the game Hitman: Contracts, the player can assassinate people using cyanide.
- In the 1999 Midsomer Murders episode "Judgement Day", the second murder victim is poisoned by cyanide mixed in a glass of wine.
- In the 2008 Doctor Who episode "The Unicorn and the Wasp", the Doctor is nearly poisoned by cyanide, but manages to metabolize it and detoxify himself using a combination of proteins, salt, and a shock, plus the advantage of his non-human physiology.
- In the Assassin's Creed video game series, Alan Turing was revealed to have been killed by Templars who laced an apple (which he would later eat) with cyanide in an attempt to make it look as if he had committed suicide.
- In the film "The Little Girl Who Lives Down the Lane", Rynn Jacobs (Jodie Foster) poisons Frank Hallet (Martin Sheen) with cyanide-tainted tea, serving him almond cookies to mask the taste.
- In the film Irrational Man, cyanide is used to kill a judge.
Suicide
- In Agatha Christie's novel The Hollow, woman called Gerda Christow kills herself when she gets caught by murder of her husband.
- In Agatha Christie's novel The Secret Adversary, the villain "Mr Brown" commits suicide using cyanide concealed in a signet ring.
- In Robert Louis Stevenson's Strange Case of Dr Jekyll and Mr Hyde, Dr. Jekyll kills himself with cyanide, deduced as the smell of kernels (almonds) is evident.
- In Ian Fleming's James Bond stories and the movies based on them, 00 agents are issued cyanide capsules for use in the event of capture by the enemy. James Bond is described as having thrown his away.
- Gabriel García Márquez's novel Love in the Time of Cholera begins with Jeremiah de Saint-Amour's suicide by cyanide poisoning.
- Australian author Nevil Shute's 1957 novel about life after nuclear war, On the Beach, gives the scenario of the Australian government giving survivors free cyanide tablets to commit suicide rather than face death from radiation poisoning.
- In William Styron's 1979 novel Sophie's Choice and the movie based on the book, Sophie and Nathan commit suicide by ingesting a cyanide pill.
- In Ford Maddox Ford's novel The Good Soldier one of the main characters, Florence, commits suicide by drinking her phial of "prussic acid" after learning that her lover is having an affair with another woman.
- In Japanese author Koushun Takami's 1999 novel Battle Royale and the film Battle Royale based on the book, Yuko Sakaki is given a small bottle of potassium cyanide (KCN) as a "special bonus" in addition to the weapon provided in her day pack.
- In the film Captain America: The First Avenger (2011), Heinz Kruger commits suicide by cyanide tablet upon being caught.
- In the film Unknown (2011), Jürgen commits suicide by emptying a bag of sodium cyanide into his coffee, disguised as a packet of sugar.
- In the Kannada film Cyanide (2006), which is about the incidents that occurred in the peripheries of Bangalore after the assassination of the former Indian Prime Minister Rajiv Gandhi, the killers of the Prime Minister use cyanide vials to commit suicide to avoid being captured by the police.
- In the James Bond film Dr. No (1962), James Bond believes that his cab driver is an enemy agent, and after a fight scene, begins to interrogate the driver, who proceeds to poison and kill himself with cyanide embedded in a cigarette.
- In the James Bond film Skyfall (2012), Raoul Silva discusses his failed attempt to commit suicide using a hydrogen cyanide capsule whilst under interrogation. Rather than kill him, the hydrogen cyanide burned his body internally, forcing him to wear a prosthetic face plate to hide his disfigurement.
- In the second season of the US TV series 24, several of the terrorists keep cyanide pills immediately on their person so they can swallow them immediately to avoid capture.
Other
- Isaac Asimov's short story "Hostess" features an alien race which requires small amounts of hydrogen cyanide in order for their hemoglobin analogues to remain stable. As such, while they do not suffer cyanide poisoning, cyanide withdrawal is, for them, an extremely painful condition similar to slow strangulation.
- In Erwin Schrödinger's famous thought experiment Schrödinger's cat, a flask of hydrocyanic acid is rigged to release inside a box upon the decay of a radioactive substance, randomly poisoning a cat. In such a scenario, the macroscopic effects of quantum indeterminacy would cause the cat to be neither alive nor dead until observation.
Chemistry and crime
Toxicity and Poisons
Poisons have been known from antiquity, and were used by ancient tribes and civilizations as hunting tools to quicken and ensure the death of their prey or enemies. This use of poison grew more advanced, and many of these ancient peoples began forging weapons designed specifically for poison enhancement. Later in history, particularly at the time of the Roman Empire, one of the more prevalent uses was for assassination. As early as 331 BC, poisonings executed at the dinner table or in drinks were reported, and the practice became a common occurrence. The use of fatal substances was seen among every social class; even the nobility would use it to dispose of unwanted political or economic opponents.
Arsenic.
Until the 19th century, most poisons were undetectable as well as common and this meant that poisoners could expect to escape detection and punishment. Family members or neighbours might be suspect if an unloved wife or husband or a rich parent died suddenly, but no one could prove that such a person had been poisoned. As a result, historians say, poisoning was widespread in some places and times, such as in Italy and France in the late 1600s.
The most popular poison seems to have been arsenic. The human body needs tiny amounts of this metallic element, but arsenic is poisonous in most doses. Arsenic was most commonly found in the form of arsenic oxide, a white powder that had respectable uses ranging from improving the complexion to poisoning rats. Because white arsenic, as the powder was called, was odourless and tasteless as well as easy to buy, however, some people applied it to less legitimate purposes. Secretly mixed into food, the powder caused stomach pains, vomiting, diarrhea, and other signs of illness just like the symptoms of cholera and several other common, deadly diseases. Only a minute dose of arsenic (about 0.25 g) was needed to kill a person. White arsenic was supposedly used so often to poison rich relatives in late 17th-century France that it was nicknamed "inheritance powder".
Mathieu Orfila, born 1787 in Minorca, is considered the father of toxicology. In 1813, not long after he graduated as a doctor, he was living in Paris and giving private lessons in Chemistry to help cover his bills. After he failed several times to show his students the precipitate that was claimed to form when arsenic acid was mixed with various substances - a common test for arsenic at the time - he decided to examine other standard tests for poisons in fluids such as soup, wine, and coffee. He found that most of the tests were unreliable. He learnt that the detection of many poisons was achieved by feeding suspected substances to animals and waiting to see whether the animals died. Orfila went on to create new techniques and refined existing techniques in his first treatise, Traité des poisons, greatly enhancing their accuracy. The book divided poisons into several groups and described their effects on the living body, the symptoms of illness they produce, the signs they leave in a dead body, and the ways of identifying them. The first volume of this exhaustive work appeared in 1813, and a second volume in 1815.

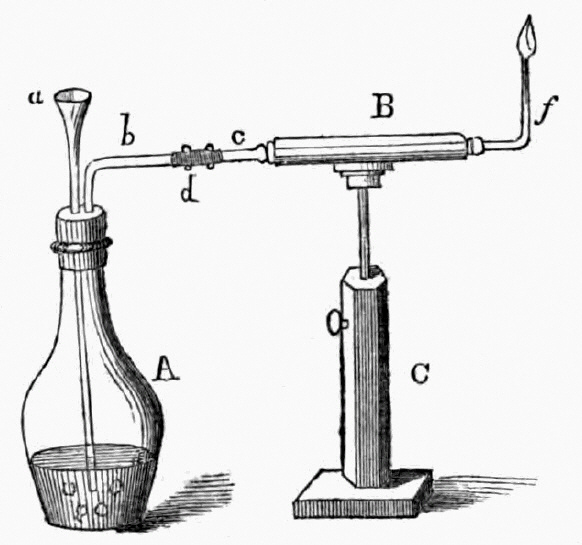
1818 publication by Orfila and Marsh apparatus for the detection of arsenic
a= funnel, b= escape tube, C= stand
In 1832, James Marsh was called as a chemist by the prosecution in a murder trial, wherein a certain John Bodle was accused of poisoning his grandfather with arsenic-laced coffee. Marsh performed the standard test by mixing a suspected sample with hydrogen sulfide and hydrochloric acid. While he was able to detect arsenic as yellow arsenic trisulfide, when it came to show it to the jury it had deteriorated, allowing the suspect to be acquitted due to reasonable doubt. Annoyed by this, Marsh developed a much better test. The material to be tested was mixed with zinc and sulfuric acid in the flask at the left (A). If the sample contained arsenic, hydrogen from the acid combined with the arsenic to form arsine gas. The gas passed into the horizontal calcium chloride drying tube (B). Near the end of the tube, the gas was heated by a flame (f). The heat broke down the arsine and released metallic arsenic, that formed a black, shiny deposit (called the arsenic mirror) at the end of the tube. So sensitive was the test that it could detect as little as one-fiftieth of a milligram of arsenic.
Marie-Fortunée Lafarge, née Capelle (January 15, 1816 - November 7, 1852) was a Frenchwoman said to be a descendant of Louis XIII (27 Sept 1601-14 May 1643) of France through her grandmother. She gained notoriety in 1840 since she was convicted of murdering her husband by arsenic poisoning. Her case became notable, because it was one of the first trials to be followed by the public through daily newspaper reports, and because she was the first person convicted largely on direct forensic toxicological evidence.
After several tests had been carried out by local chemists, Orfila was called to perform the Marsh Test at the courthouse in the presence of the local chemists. He confirmed the presence of arsenic in the deceased's body as well as food material gathered from the household. Marie was convicted and given a life-sentence.
See as well the article on arsenic detection and the Moreau and LaFarge cases.
Arsenic in drinking water
The World Health Organisation (WHO) notes that Arsenic in drinking-water is a hazard to human health. The contamination of groundwater by arsenic in Bangladesh is the largest poisoning of a population in history. With a total population of around 125 million, they estimated that between 35-77 million people were at risk from exposure. It occurs less extensively in many other countries as well. Studies have shown that where the population has had long-term exposure to arsenic in groundwater 1 in 10 people who drink water containing 500 mg of arsenic per litre may ultimately die from cancers caused by arsenic, including lung, bladder and skin cancers.
| Population of Bangladesh | 125 million |
| Total population in regions where some wells are known to be contaminated | 35-77 million |
| Maximum concentration of arsenic permitted in drinking-water according to WHO recommendations | 10 mg/l |
| Maximum concentration allowed in Bangladesh | 50 mg/l (similar to many countries worldwide) |
| Number of tube-wells sampled by the British Geological Survey (1998) | 2022 |
| Proportion of wells with arsenic concentrations >50 mg/l | 35% |
| Proportion of wells with arsenic concentrations >300 mg/l | 8.4% |
In the USA, the EPA is required by Congress (1974 Safe Drinking Water Act) to determine the level of contaminants in drinking water at which no adverse health effects are likely to occur. These non-enforceable health goals, based solely on possible health risks and exposure over a lifetime with an adequate margin of safety, are called maximum contaminant level goals (MCLG).
Based on the MCLG (for As = ZERO), the EPA has set an enforceable regulation called a maximum contaminant level (MCL), at 0.010 mg/L or 10 ppb. MCLs are set as close to the health goals as possible, considering cost, benefits and the ability of public water systems to detect and remove contaminants using suitable treatment technologies.
Approximately 90 percent of industrial arsenic used in the U.S. is as a wood preservative, but arsenic is found in paints, dyes, metals, drugs, soaps, and semi-conductors as well. Agricultural applications, mining, and smelting can contribute to arsenic releases in the environment. The major sources of arsenic in drinking water are erosion of natural deposits; runoff from orchards; and runoff from glass and electronics production wastes.
Recently a group in Florida have devised a method for As removal from drinking water using a "Biochar" filter method. A magnetic, porous biochar absorbent was prepared from the thermal pyrolysis of FeCl3 treated biomass. Batch sorption experimental results showed that the composite had strong sorption abilities towards aqueous arsenic. Due to its excellent ferromagnetic properties, the arsenic-laden biochar/γ-Fe2O3 composite could be readily separated from the solution by a magnet at the end of the sorption experiment.
A November 2014 follow-up report on iron-enhanced carbon cooked from hickory chips noted that ground wood chips were heated in nitrogen gas, but not burned. The resulting biochar, that had the consistency of ground coffee, was then treated with a saltwater bath to impregnate it with iron. Homeowners might eventually be able to use a small filter attached to their tap.
Common poisonous cyanide compounds include hydrogen cyanide gas and the crystalline solids potassium cyanide and sodium cyanide. The cyanide ion halts cellular respiration by inhibiting an enzyme in the mitochondria called cytochrome c oxidase.
John Tawell (1784-1845) was a British murderer who was eventually hanged in public in front of a crowd of around 10,000 people. In 1845, he became the first person to be arrested as the result of telecommunications technology. It was also the first known homicide case where the criminal attempted to flee the scene of the crime by a railway train.
Cyanide compounds are found naturally in the stones of apricots, cherries, plums, peaches, and the cores of apples where it may have evolved as a plant protection mechanism against grazing animals. A number of bacteria, fungi and algae are able to produce cyanide as well. Ingestion of moderate amounts of these natural substances causes headaches accompanied by mild heart palpitations, providing a warning that they should be avoided. Middle Eastern people of ancient times made the discovery that the distillation, by evapouration of laurel leaves, produced lethal concentrations of this innocent plant product.
Some millipedes release HCN as a defense mechanism and certain insects such as day-flying moths contain a deadly dose of cyanide to deter predators. Cassava root contains linamarin, a natural molecule containing both glucose and cyanine groupings and perhaps the cyanide is present to protect the plant, although since we recognise that cassava is the third-largest source of food carbohydrates in the tropics, it is no longer working very well against humans!!
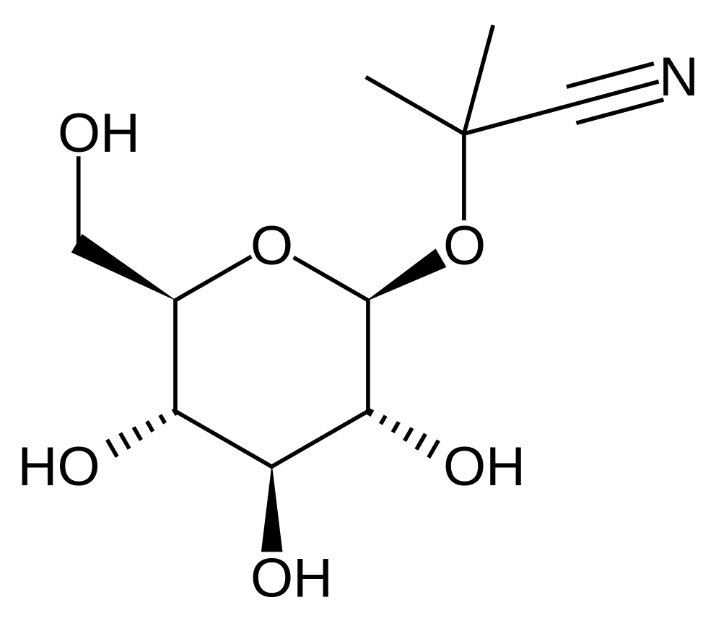 | linamarin |
Cassava is a major staple food in the developing world, providing a basic diet for around 502 million people, some eating as much as 0.5 kg a day in the form of porridge. It is one of the most drought-tolerant crops, capable of growing on marginal soils. Nigeria is the world's largest producer of cassava.
Cassava is prepared by peeling and grating the root, soaking it in water for at least 3 days, drying in the sun and finally grinding it into a flour that can be used to make the porridge. Too much though can give rise to a disease called konzo.
The onset of paralysis (hypertonic paraparesis) is sudden and symmetrical and if the symptoms do not disappear within a few days the resulting disability is permanent, but does not progress. The disease onset is associated with high dietary exposure from cyanide liberated from the naturally occurring glucosides that normally are removed by processing (washing and drying) before consumption of bitter cassava roots. However, during food shortage, war and other severe disruptions of life in poor rural cassava growing communities, the population is forced to make short-cuts in the established procedures for removing the cyanide.
Fresh cassava root contains 300 mg of cyanide per kg, but if it is peeled and soaked in water for ONE day then sun-dried for four days, this falls to 80 mg per kg. If peeled and soaked for FIVE days and then sun-dried for a week this figure is further reduced to 30 mg per kg. Grating before soaking can reduce this amount even further to 10 mg per kg and this is the value recommended by the WHO as the MAXIMUM limit for cyanide in cassava flour. This value was chosen so that anyone eating 500 g of cassava would still be one tenth the estimated lethal dose of around 50 mg.
Cyanide has been used in gold-mining industry and even more exotically by butterfly collectors (who use NaCN - in their collection bottles so that the butterfies die quickly without much fluttering of wings), its most notable use throughout history was as a poison. One of the first administrators of cyanide was said to be Livia, the wife of Augustus who, in AD 14 killed her husband by soaking his figs in the poison. A number of people elected to end their lives by cyanide including: Adolf Hitler, Eva Braun, Hermann Goering, Wallace Carothers and Alan Turing.
Some people claim that cyanide has a sweet, sickly almond smell. People who have been exposed to the poison sometimes describe a faint bitter almond taste in the breath and stomach - a sure sign of cyanide poisoning. Note though that there are some people who cannot smell cyanide at all, due to a genetic trait. However it smells, its actions are brutal and deadly; as little as 50 mg causes death by anoxia within five minutes.
Symptoms will be slow to reveal in the case of chronic poisoning, and may include general weakness, confusion, bizarre behaviour, excessive sleepiness, shortness of breath, dizziness, headache and seizures. If a large dose is taken at one go, the heart will immediately be affected, causing a sudden collapse; the brain may also be affected, causing a seizure or a coma. Death follows rapidly as the poison prevents oxygen from reaching the cells. Post mortem will reveal healthy lungs whose coverings show signs of inflammation, and the skin of a poisoned victim may sometimes be pink or cherry-red in colour as oxygen remains in the blood.
The extent of a person's exposure to cyanide can be assessed by determining the amount of thiocyanate in their urine, since this is a metabolic product in the bodies attempt at detoxification by the liver. Generally SCN- is present at under 6 mg per litre but in villages affected by konzo, for example, the level may reach 60 mg per litre.
The toxicity of cyanide is due to it's binding to iron in the enzyme, cytochrome oxidase which is present in the membrane of cell mitochondria. This enzyme is critical in the oxidation of glucose by di-oxygen, and is vital for the generation of energy. Without it the energy processes cannot proceed and so life ends very quickly. The heart and central nervous system are immediately affected and because the oxygen being transported by the haemoglobin is not used up a symptom of cyanide poisoning is that those affected often show the bright red colour of oxygenated blood.
The seeds and bark of many plants of the genus Strychnos, Family Loganiaceae and in particular the strychnine tree (Strychnos nux-vomica L.) contains the powerful poison strychnine. The seeds contain approximately 1.5% strychnine, and the dried blossoms contain 1.0%. However, the tree's bark contains brucine and other poisonous compounds.
Strychnine is a highly toxic colourless crystalline alkaloid used for years to get rid of rodents and birds. In September 2006 the EU banned its use for killing moles, as it caused them unnecessary suffering.
Death by strychnine must truly be one of the most horrific ways to die. It begins with a general feeling of restlessness and a feeling of impending suffocation. As the poison spreads through the body, the facial muscles contract, and the face is drawn into a gruesome characteristic grin called risus sardonicus. Other muscles of the body will subsequently become similarly affected, causing the body to be violently and spasmodically jerked into all sorts of contortions - bent backward like a bow one minute, with the head and heels resting on the surface (a condition known as opisthotonos), and twisted in the other direction off the bed in the next. These paroxysms will last for several minutes, after which there is a period of relative quiet, during which time the victim will often complain of exhaustion and great thirst. But this is no sign of convalescence, for the next attack begins right after, and even more violently. The stomach muscles will harden and tense, the face will grow livid, with the jaws clenched shut in the fashion of lockjaw; observers will perceive the victim's eyeballs to be staring and prominent in a disturbing manner. Through all this agony, the victim remains fully conscious of the unravelling horrors.
It operates by blocking a glycine receptor that stops motor nerves in the spinal cord from operating normally. It heightens sensitivity to stimuli, resulting in excessive muscular contractions. Death is a result of either suffocation by paralysis of breathing, or of exhaustion from the continuous convulsions. Since strychnine does not cross the blood-brain barrier, the victim remains conscious throughout and because the symptoms are enhanced by noise and light, victims must be kept quiet to ease their suffering.
Plants are brilliant synthetic organic chemists, and the Strychnos nux vomica tree is one of the most talented, as strychnine has a fiendishly complicated structure, containing seven rings fused together. The plant makes it from the amino acid tryptophan; no one is sure why, though this toxic and bitter-tasting molecule could be a deterrent to predators.
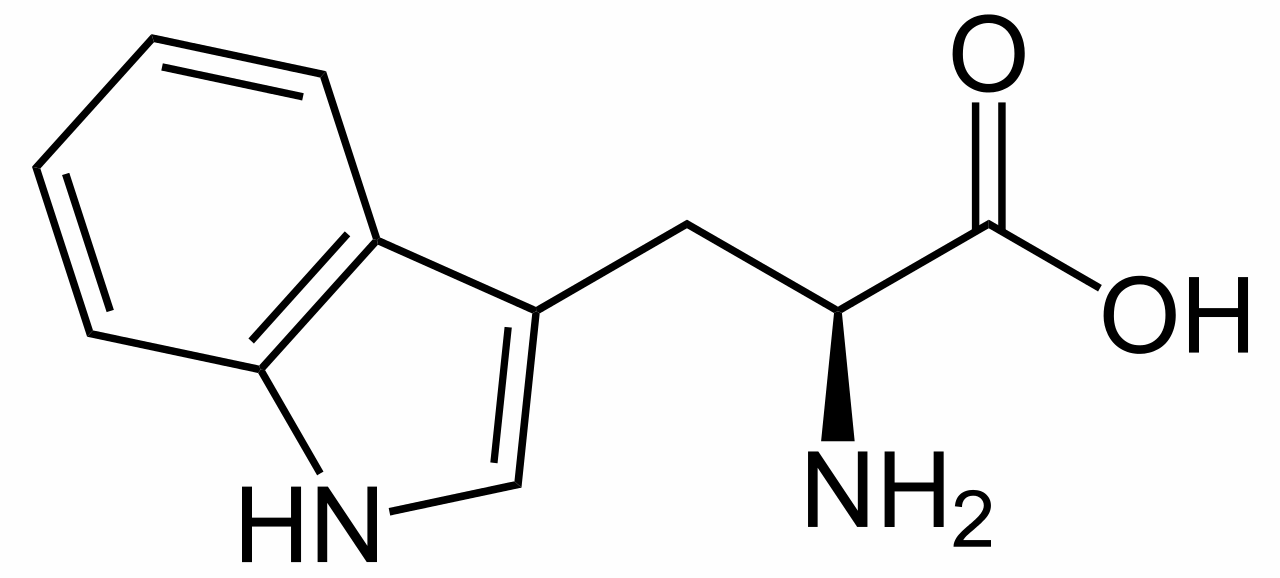  L-tryptophan is the biosynthetic precursor to strychnine |
The toxic and medicinal effects of strychnine have been well known from the times of ancient China and India. In Europe, historic records indicate that the strychnine alkaloid had been used to kill dogs, cats, and birds as far back as 1640. The structure of strychnine was first determined in 1946 by Sir Robert Robinson and in 1954 the first total synthesis by Robert B. Woodward was reported. This is one of the most famous syntheses in the history of organic chemistry. Both chemists won the Nobel prize (Robinson in 1947 and Woodward in 1965). In a 1963 publication, Woodward quoted Sir Robert Robinson who said "for its molecular size, it is the most complex substance known".
| Toxic Compound | Occurrence | Type | ~RMM | LD50 * |
|---|---|---|---|---|
| Most Toxic | ||||
| maitotoxin | dinoflagellum | polyketide | 3422 | 0.050 |
| ciguatoxin | dinoflagellum | polyketide | 1061 | 0.35 |
| palytoxin | coral species | polyketide | 2679 | 0.45 |
| taipoxin | Australian taipan snake | glycoprotein | 45600 | 2 |
| batrachotoxin | Columbian poison dart frog | steroid alcohol | 539 | 2 |
| tetrodotoxin | pufferfish | saccharide derivative | 319 | 10 |
| Plant poisons | ||||
| ricin | castor bean | glycoprotein (lectin) | 62400 | 0.1 |
| nicotine | tobacco plant | alkaloid | 162 | 300 |
| strychnine | poison nut | alkaloid | 334 | 750 |
| cymarine | tropical creeping shrub | digitalis glycoside | 549 | 25000 |
| tubocurarine chloride | tropical woody vine | alkaloid | 682 | 33200 |
| atropine | deadly nightshade | alkaloid | 289 | 400000 |
| Fungal poisons | ||||
| L-(+)-muscarine | "fly agaric" | alkaloid | 174 | 230 |
| α-amanitin | "death cap" | bicyclic octapeptide | 919 | 300 |
| penitrem A | mould | polycyclic indole derivative | 634 | 1050 |
| aflatoxin b1 | mould | difuran coumarin derivative | 312 | 1700 |
| Inorganic and synthetic poisons | ||||
| 2,3,7,8-TCDD (dioxin) | polychlorinated dibenzo-p-dioxin | 320 | 22 | |
| parathion (E605) | organophoshate | 291 | 3600 | |
| potassium cyanide | 66 | 10000 | ||
| arsenic oxide | 198 | 15100 | ||
* LD50 generally given as μg kg-1
Animal derived toxins
The reef stonefish, or simply stonefish, is a fish species (Synanceia verrucosa) that is sometimes lethal to humans. They are carnivorous ray-finned fish with venomous spines that live on reef bottoms, camouflaged as a rock.
The Reef Stonefish is the most venomous fish in the world. Its dorsal area is lined with 13 spines that release venom from two sacs attached to each spine. Its venom causes severe pain with possible shock, paralysis, and tissue death depending on the depth of the penetration. This level can be fatal to humans if not given medical attention within a couple of hours. Immediate first aid treatment requires immersion of the affected limb in hot water, ensuring that it is not so hot that skin damage may occur.
The venom consists of a mixture of proteins, including the hemolytic stonustoxin, the protinaceous verrucotoxin and the cardioactive cardioleputin; an antivenom is available.
The red lionfish (Pterois volitans) is a venomous coral reef fish in the family Scorpaenidae, order Scorpaeniformes. P. volitans is natively found in the Indo-Pacific region, but has become a huge invasive problem in the Caribbean Sea and along the East coast of the United States along with a similar species, Pterois miles. Red lionfish are clad in white stripes alternated with red, maroon, or brown. Adults can grow as large as 43 cm (17 inches) in length while juveniles may be shorter than 2.5 cm (1 inch). They can live up to 10 years. The fish has large venomous spines that protrude from the body like a mane, giving it the common name of the lionfish. The venomous spines make the fish inedible or deter most potential predators. Lionfish reproduce monthly and are able to quickly disperse during their larval stage for expansion of their invasive region. There are no definitive predators of the lionfish, and many organizations are promoting the harvest and consumption of lionfish in efforts to prevent further increases in the already high population densities.

Lionfish
The most distinguishable characteristic of the red lionfish, as well as all scorpionfishes, are the venomous spines protruding from the body. An extremely showy and ornate fish such as the lionfish should be an easy target for predators, but the large spines act as a great defense. The spines are incorporated into certain fins of the fish, and have venom glands at the base of the spine. These glands protect the fish from predation, delivering a painful and potentially fatal venomous "sting" to predators or a human that may come in contact with a lionfish.
Tetraodontidae is a family of primarily marine and estuarine fish of the Tetraodontiformes order. The family includes many familiar species which are variously called pufferfish, balloonfish, blowfish, bubblefish, globefish, swellfish, toadfish, toadies, honey toads, sugar toads, and sea squab. They are morphologically similar to the closely related porcupinefish, which have large external spines (unlike the thinner, hidden spines of Tetraodontidae, which are only visible when the fish has puffed up). The scientific name refers to the four large teeth, fused into an upper and lower plate, which are used for crushing the shells of crustaceans and mollusks, their natural prey.
(Maple) Puffer fish are generally believed to be the second-most poisonous vertebrate in the world, after the Golden Poison Frog. Certain internal organs, such as liver, and sometimes their skin are highly toxic to most animals when eaten, but nevertheless the meat of some species is considered a delicacy in Japan (Fugu), Korea and China when prepared by chefs who know which part is safe to eat and in what quantity. Although the liver is considered to have the best taste, it was found to be the most toxic so has been banned in restaurants in Japan since 1984.
Pufferfish can be lethal if not served properly. Puffer poisoning usually results from consumption of incorrectly prepared puffer soup, fugu chiri, or occasionally from raw puffer meat, sashimi fugu. While chiri is much more likely to cause death, sashimi fugu often causes intoxication, light-headedness, and numbness of the lips, and is often eaten for this reason. Puffer's (tetrodotoxin) poisoning deadens the tongue and lips, and induces dizziness and vomiting, followed by numbness and prickling over the body, rapid heart rate, decreased blood pressure, and muscle paralysis. The toxin paralyzes diaphragm muscles and stops the person who has ingested it from breathing. People who live longer than 24 hours typically survive, although possibly after a coma lasting several days. Some people claim to have remained fully conscious throughout the coma, and can often recount events that occurred while they were supposedly unconscious. The paralysis reduces oxygen demands of the body dramatically, but because the toxin does not cross the blood-brain barrier, neural activity in the brain and from the eyes and ears are generally intact. In Voodoo, puffer's poison may be part of the mixture given to the victim to make them a "zombie", most likely because the paralysis and pseudo-comatose effect simulate the death portion of traditional zombie creation.
 | tetrodotoxin |
Although tetrodotoxin was discovered in these fish and found in several other animals (e.g., blue-ringed octopus, rough-skinned newt, and Naticidae) it is now believed that puffers do NOT produce toxins themselves, as puffer fish kept in tanks or fish farms are totally free of the toxin. The toxin is now believed to be produced by certain symbiotic bacteria, such asPseudoalteromonas tetraodonis, certain species of Pseudomonas and Vibrio, as well as some others that reside within these animals. The gastric contents of shellfish prey are believed to carry the toxins or their precursors, which are stored in the puffers organs.
Saxitoxin, the cause of paralytic shellfish poisoning and red tide has also been found in certain puffers.
A recent study involving "spy-cams" disguised as turtles has found that young dolphins can antagonise puffer-fish so that they release the toxin and this has a narcotic effect on the dolphins.
Poison dart frog (also dart-poison frog, poison frog or formerly poison arrow frog) is the common name of a group of frogs in the family Dendrobatidae which are native to Central and South America. These species are diurnal and often have brightly-colored bodies. Although all wild dendrobatids are at least somewhat toxic, levels of toxicity vary considerably from one species to the next and from one population to another. Many species are critically endangered. These amphibians are often called "dart frogs" due to the Amerindians' indigenous use of their toxic secretions to poison the tips of blowdarts. However, of over 175 species, only three have been documented as being used for this purpose (curare plants are more commonly used), and none come from the Dendrobates genus, which is characterized by the brilliant color and complex patterns of its members.
Many poison dart frogs secrete lipophilic alkaloid toxins through their skin. Alkaloids in the skin glands of poison frogs serve as a chemical defense against predation, and they are therefore able to be active alongside potential predators during the day. About 28 structural classes of alkaloids are known in poison frogs. The most toxic of poison-dart frog species is Phyllobates terribilis. It is argued that dart frogs do not synthesize their poisons, but sequester the chemicals from arthropod prey items, such as ants, centipedes and mites. This is known as the dietary hypothesis. Because of this, captive-bred animals do not contain significant levels of toxins. Despite the toxins used by some poison dart frogs, there are some predators that have developed the ability to withstand them, including the Amazon ground snake (Liophis epinephelus).
Chemicals extracted from the skin of Epipedobates tricolor may be shown to have medicinal value. One such chemical is a painkiller 200 times as potent as morphine, called epibatidine, that has unfortunately demonstrated unacceptable gastrointestinal side effects in humans. Secretions from dendrobatids are also showing promise as muscle relaxants, heart stimulants and appetite suppressants. The most poisonous of these frogs, the Golden Poison Frog (Phyllobates terribilis), has enough toxin on average to kill ten to twenty men or about ten thousand mice. Most other dendrobatids, while colorful and toxic enough to discourage predation, pose far less risk to humans or other large animals.
epibatidine and batrachotoxin |
Plant derived toxins
Ricin is produced by the castor bean plant Ricinus communis. It makes this highly toxic, naturally occurring protein in its seeds, possibly as a deterrent to predators, since once the seed has germinated the toxin disappears. The LD50 for ricin in rodents is listed as 0.1 microgram/kilogram and for humans as 20 microgram/kilogram.
 | |
| Ricin is an example of an holotoxin, consisting of heterodimeric glycoproteins, the A and B chains. | |
The ricin molecule consists of two long chains (A and B) neither of which are considered toxic by themselves. The A chain for example can be found in barley. The B chain is the critical component since it is able to bind to the outside of the cell membrane via a carbohydrate compone.
POTASSIUM CYANIDE : Systemic Agent
Common Names:
- Potassium salt of hydrocyanic acid
Agent Characteristics
- APPEARANCE: White, granular or crystalline solid.
- DESCRIPTION: Potassium cyanide releases hydrogen cyanide gas, a highly toxic chemical asphyxiant that interferes with the body's ability to use oxygen. Exposure to potassium cyanide can be rapidly fatal. It has whole-body (systemic) effects, particularly affecting those organ systems most sensitive to low oxygen levels: the central nervous system (brain), the cardiovascular system (heart and blood vessels), and the pulmonary system (lungs). Potassium cyanide is used commercially for fumigation, electroplating, and extracting gold and silver from ores. Hydrogen cyanide gas released by potassium cyanide has a distinctive bitter almond odor (others describe a musty "old sneakers smell"), but a large proportion of people cannot detect it; the odor does not provide adequate warning of hazardous concentrations. It is usually shipped as capsules, tablets, or pellets. Potassium cyanide absorbs water from air (is hygroscopic or deliquescent).
- METHODS OF DISSEMINATION:
- Indoor Air: Potassium cyanide can be released into indoor air as fine droplets, liquid spray (aerosol), or fine particles.
- Water: Potassium cyanide can be used to contaminate water.
- Food: Potassium cyanide can be used to contaminate food.
- Outdoor Air: Potassium cyanide can be released into indoor air as fine droplets, liquid spray (aerosol), or fine particles.
- Agricultural: If potassium cyanide is released as fine droplets, liquid spray (aerosol), or fine particles, it has the potential to contaminate agricultural products.
- ROUTES OF EXPOSURE: Potassium cyanide can affect the body by ingestion, inhalation, skin contact, or eye contact.
- GENERAL INFORMATION: First Responders should use a NIOSH-certified Chemical, Biological, Radiological, Nuclear (CBRN) Self Contained Breathing Apparatus (SCBA) with a Level A protective suit when entering an area with an unknown contaminant or when entering an area where the concentration of the contaminant is unknown. Level A protection should be used until monitoring results confirm the contaminant and the concentration of the contaminant.NOTE: Safe use of protective clothing and equipment requires specific skills developed through training and experience.
- LEVEL A: (RED ZONE): Select when the greatest level of skin, respiratory, and eye protection is required. This is the maximum protection for workers in danger of exposure to unknown chemical hazards or levels above the IDLH or greater than the AEGL-2.
- A NIOSH-certified CBRN full-face-piece SCBA operated in a pressure-demand mode or a pressure-demand supplied air hose respirator with an auxiliary escape bottle.
- A Totally-Encapsulating Chemical Protective (TECP) suit that provides protection against CBRN agents.
- Chemical-resistant gloves (outer).
- Chemical-resistant gloves (inner).
- Chemical-resistant boots with a steel toe and shank.
- Coveralls, long underwear, and a hard hat worn under the TECP suit are optional items.
- LEVEL B: (RED ZONE): Select when the highest level of respiratory protection is necessary but a lesser level of skin protection is required. This is the minimum protection for workers in danger of exposure to unknown chemical hazards or levels above the IDLH or greater than AEGL-2. It differs from Level A in that it incorporates a non-encapsulating, splash-protective, chemical-resistant splash suit that provides Level A protection against liquids but is not airtight.
- A NIOSH-certified CBRN full-face-piece SCBA operated in a pressure-demand mode or a pressure-demand supplied air hose respirator with an auxiliary escape bottle.
- A hooded chemical-resistant suit that provides protection against CBRN agents.
- Chemical-resistant gloves (outer).
- Chemical-resistant gloves (inner).
- Chemical-resistant boots with a steel toe and shank.
- Coveralls, long underwear, a hard hat worn under the chemical-resistant suit, and chemical-resistant disposable boot-covers worn over the chemical-resistant suit are optional items.
- LEVEL C: (YELLOW ZONE): Select when the contaminant and concentration of the contaminant are known and the respiratory protection criteria factors for using Air Purifying Respirators (APR) or Powered Air Purifying Respirators (PAPR) are met. This level is appropriate when decontaminating patient/victims.
- A NIOSH-certified CBRN tight-fitting APR with a canister-type gas mask or CBRN PAPR for air levels greater than AEGL-2.
- A NIOSH-certified CBRN PAPR with a loose-fitting face-piece, hood, or helmet and a filter or a combination organic vapor, acid gas, and particulate cartridge/filter combination or a continuous flow respirator for air levels greater than AEGL-1.
- A hooded chemical-resistant suit that provides protection against CBRN agents.
- Chemical-resistant gloves (outer).
- Chemical-resistant gloves (inner).
- Chemical-resistant boots with a steel toe and shank.
- Escape mask, face shield, coveralls, long underwear, a hard hat worn under the chemical-resistant suit, and chemical-resistant disposable boot-covers worn over the chemical-resistant suit are optional items.
- LEVEL D: (GREEN ZONE): Select when the contaminant and concentration of the contaminant are known and the concentration is below the appropriate occupational exposure limit or less than AEGL-1 for the stated duration times.
- Limited to coveralls or other work clothes, boots, and gloves.
- CHEMICAL DANGERS:
- Potassium cyanide is water-reactive.
- Potassium cyanide decomposes on contact with water, humidity, carbon dioxide, and acids, producing very toxic and highly flammable hydrogen cyanide gas.
- Potassium cyanide solution in water is a strong base; it reacts violently with acid and is corrosive.
- Potassium cyanide undergoes violent chemical reactions with chlorates and nitrites.
- EXPLOSION HAZARDS:
- Chlorates plus potassium cyanide explode when heated.
- A mixture of potassium cyanide and nitrites may cause an explosion.
- Nitrogen chloride explodes on contact with potassium cyanide.
- Containers may explode when heated or if they are contaminated with water.
- Upper and lower explosive (flammable) limits in air are not available for potassium cyanide.
- FIRE FIGHTING INFORMATION:
- Potassium cyanide is non-combustible.
- The agent itself does not burn, but it may decompose upon heating to produce corrosive and/or toxic fumes.
- Water-sensitive: Potassium cyanide releases highly flammable and toxic hydrogen cyanide gas on contact with water or damp air and in a fire.
- Note: Most foams will react with the agent and release corrosive/toxic gases.
- For small fires, do not use carbon dioxide; use dry chemical, dry sand, or alcohol-resistant foam.
- For large fires, use water spray, fog, or alcohol-resistant foam. Move containers from the fire area if it is possible to do so without risk to personnel. Use water spray or fog; do not use straight streams. Dike fire control water for later disposal; do not scatter the material.
- For fire involving tanks or car/trailer loads, fight the fire from maximum distance or use unmanned hose holders or monitor nozzles. Do not get water inside containers. Cool containers with flooding quantities of water until well after the fire is out. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tanks. Always stay away from tanks engulfed in fire.
- Run-off from fire control or dilution water may be corrosive and/or toxic, and it may cause pollution.
- If the situation allows, control and properly dispose of run-off (effluent).
- INITIAL ISOLATION AND PROTECTIVE ACTION DISTANCES:
- If a tank, rail car, or tank truck is involved in a fire, isolate it for 0.5 mi (800 m) in all directions; also consider initial evacuation for 0.5 mi (800 m) in all directions.
- Small spills (when spilled in water)
- First isolate in all directions: 100 ft (30 m).
- Then protect persons downwind during the day: 0.1 mi (0.1 km).
- Then protect persons downwind during the night: 0.3 mi (0.5 km).
- Large spills (when spilled in water)
- First isolate in all directions: 1000 ft (300 m).
- Then protect persons downwind during the day: 0.6 mi (1.0 km).
- Then protect persons downwind during the night: 2.4 mi (3.9 km).
- PHYSICAL DANGERS:
- Vapors may collect and stay in confined areas (e.g., sewers, basements, and tanks).
- Hazardous concentrations may develop quickly in enclosed, poorly-ventilated, or low-lying areas. Keep out of these areas. Stay upwind.
- Hydrogen cyanide gas produced from potassium cyanide mixes well with air; explosive mixtures are easily formed.
- NFPA 704 Signal:
- Health: 3
- Flammability: 0
- Reactivity: 0
- Special:

- SAMPLING AND ANALYSIS:
- OSHA: ID120
- NIOSH: 6010, 7904
- ADDITIONAL SAMPLING AND ANALYSIS INFORMATION:References are provided for the convenience of the reader and do not imply endorsement by NIOSH.
- AIR MATRIXNIOSH [1994]. NMAM Method 7904: Cyanides, aerosol and gas. In: NIOSH manual of analytical methods. 4th ed. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 94-113.
- OTHERNo references were identified for this sampling matrix for this agent.
- SOIL MATRIXEPA [1996]. SW-846 Method 9013: Cyanide extraction procedure for solids and oils. Washington, DC: U.S. Environmental Protection Agency.Matsumura M, Kojima T [2003]. Elution and decomposition of cyanide in soil contaminated with various cyanocompounds. J Hazard Materials 97(1-3):99-110.
- SURFACESZhang XG, Li HY, Ma CS, Wang XY, Bai JL, He GZ, Lou NQ [1997]. Structures and formation mechanism of potassium cyanide clusters. Chem Phy Lett 274(1-3):115-119.
- WATERCalafat AM, Stanfill SB [2002]. Rapid quantitation of cyanide in whole blood by automated headspace gas chromatography. J Chromatogr B: Anal Technol Biomed Life Sci 772(1):131-137.Cruz-Landeira A, Lopez-Rivadulla M, Concheiro-Carro L, Fernandez-Gomez P, Tabernero-Duque MJ [2000]. A new spectrphotometric method for the toxicological diagnosis of cyanide poisoning. J Anal Toxicol 24(4):266-270.EPA [1996]. SW-846 Method 9010B: Total and amenable cyanide: distillation. Washington, DC: U.S. Environmental Protection Agency.EPA [1996]. SW-846 Method 9012B: Total and amenable cyanide (automated colorimetric, with off-line distillation). Washington, DC: U.S. Environmental Protection Agency.EPA [1996]. SW-846 Method 9014: Titrimetric and manual spectrophotometric determinative methods for cyanide. Washington, DC: U.S. Environmental Protection Agency.Franson MAH, ed. [1985]. Cyanides, Standard Methods for the examination of water and wastewater. 16th ed. Washington, DC: American Public Health Association.Rodriguez M, Sanders CA, Greenbaum E [2002]. Biosensors for rapid monitoring of primary-source drinking water using naturally occurring photosynthesis. Biosens Bioelectron 17(10):843-849.Thomson L, Anderson RA [1980]. Determination of cyanide and thiocyanate in biological fluids by gas chromatography—mass spectrometry. J Chromatogr A 188(2):357-362.Tsuge K, Kataoka M, Seto Y [2001]. Rapid determination of cyanide and azide in beverages by microdiffusion spectrophotometric method. J Anal Toxicol 25(4):228-236.Vesey CJ, McAllister H, Langford RM [1999]. A simple, rapid and sensitive semimicro method for the measurement of cyanide in blood. Ann Clin Biochem 36(6):755-758.
- TIME COURSE: Effects occur rapidly following exposure to potassium cyanide. Inhalation exposure to hydrogen cyanide gas released from potassium cyanide produces symptoms within seconds to minutes; death may occur within minutes.
- EFFECTS OF SHORT-TERM (LESS THAN 8-HOURS) EXPOSURE: Early symptoms of cyanide poisoning include lightheadedness, giddiness, rapid breathing, nausea, vomiting (emesis), feeling of neck constriction and suffocation, confusion, restlessness, and anxiety. Accumulation of fluid in the lungs (pulmonary edema) may complicate severe intoxications. Rapid breathing is soon followed by respiratory depression/respiratory arrest (cessation of breathing). Severe cyanide poisonings progress to stupor, coma, muscle spasms (in which head, neck, and spine are arched backwards), convulsions (seizures), fixed and dilated pupils, and death. The CNS is the most sensitive target organ of cyanide poisoning. Cardiovascular effects require higher cyanide doses than those necessary for CNS effects. In serious poisonings, the skin is cold, clammy, and diaphoretic. Blue discoloration of the skin may be a late finding. Severe signs of oxygen deprivation in the absence of blue discoloration of the skin suggest cyanide poisoning.
- EYE EXPOSURE:
- Redness, pain, severe burns, and tissue damage (ulceration).
- Contact with the eyes can contribute to whole-body (systemic) toxicity. See Inhalation Exposure.
- INGESTION EXPOSURE:
- Nausea, vomiting (emesis), abdominal pain, and irritation or corrosion of the lining of the esophagus and stomach.
- Whole-body (systemic) toxicity can occur. See Inhalation Exposure.
- INHALATION EXPOSURE:
- Mild to moderate: CNS effects: headache, confusion, anxiety, dizziness, weakness (malaise), and loss of consciousness. Cardiovascular effects: palpitations. Respiratory effects: respiratory tract irritation, difficulty breathing or shortness of breath (dyspnea), and transient increase in the rate and depth of breathing (hyperpnea). GI effects: nausea and vomiting (emesis).
- Severe: CNS effects: coma, seizures, and dilated pupils (mydriasis). Cardiovascular effects: shock, abnormal or disordered heart rhythms (dysrhythmias), critically low blood pressure, and cardiac arrest. Respiratory effects: abnormally rapid, followed by abnormally slow respirations; accumulation of fluid in the lungs (pulmonary edema); and respiratory arrest. Eye effects: dilated pupils, inflammation of the surface of the eye, and temporary blindness.
- SKIN EXPOSURE:
- Irritation, tissue damage (ulceration), burning sensation, and pain.
- Absorption through the skin can contribute to whole-body (systemic) toxicity. See Inhalation Exposure.
- INTRODUCTION: The purpose of decontamination is to make an individual and/or their equipment safe by physically removing toxic substances quickly and effectively. Care should be taken during decontamination, because absorbed agent can be released from clothing and skin as a gas. Your Incident Commander will provide you with decontaminants specific for the agent released or the agent believed to have been released.
- DECONTAMINATION CORRIDOR: The following are recommendations to protect the first responders from the release area:
- Position the decontamination corridor upwind and uphill of the hot zone. The warm zone should include two decontamination corridors. One decontamination corridor is used to enter the warm zone and the other for exiting the warm zone into the cold zone. The decontamination zone for exiting should be upwind and uphill from the zone used to enter.
- Decontamination area workers should wear appropriate PPE. See the PPE section of this card for detailed information.
- A solution of detergent and water (which should have a pH value of at least 8 but should not exceed a pH value of 10.5) should be available for use in decontamination procedures. Soft brushes should be available to remove contamination from the PPE. Labeled, durable 6-mil polyethylene bags should be available for disposal of contaminated PPE.
- INDIVIDUAL DECONTAMINATION: The following methods can be used to decontaminate an individual:
- Decontamination of First Responder:
- Begin washing PPE of the first responder using soap and water solution and a soft brush. Always move in a downward motion (from head to toe). Make sure to get into all areas, especially folds in the clothing. Wash and rinse (using cold or warm water) until the contaminant is thoroughly removed.
- Remove PPE by rolling downward (from head to toe) and avoid pulling PPE off over the head. Remove the SCBA after other PPE has been removed.
- Place all PPE in labeled durable 6-mil polyethylene bags.
- Decontamination of Patient/Victim:
- Remove the patient/victim from the contaminated area and into the decontamination corridor.
- Remove all clothing (at least down to their undergarments) and place the clothing in a labeled durable 6-mil polyethylene bag.
- Thoroughly wash and rinse (using cold or warm water) the contaminated skin of the patient/victim using a soap and water solution. Be careful not to break the patient/victim’s skin during the decontamination process, and cover all open wounds.
- Cover the patient/victim to prevent shock and loss of body heat.
- Move the patient/victim to an area where emergency medical treatment can be provided.
- GENERAL INFORMATION: Careful observation, supplemental oxygen, and supportive care may be sufficient therapy for the patient/victim who does not exhibit physical findings of cyanide toxicity. For the patient/victim exhibiting physical findings of cyanide toxicity, initial treatment consists of administration of antidotes under a physician's direction, respiratory and circulatory support (oxygen and IV fluids), correction of chemical imbalances in the blood, and seizure control. Speed is critical. Avoid mouth-to-mouth resuscitation regardless of route of exposure. Avoid contact with vomitus, which may off-gas hydrogen cyanide.
- ANTIDOTE: Amyl nitrite, sodium nitrite, and sodium thiosulfate are antidotes for cyanide toxicity; however, amyl nitrite and sodium nitrite should not be administered to patient/victims suffering from smoke inhalation. In these cases, only administer sodium thiosulfate. The described administration of nitrites is based on a patient having normal hemoglobin levels. Below normal hemoglobin levels require titration of nitrites.For mild to moderate physical findings such as nausea, vomiting, palpitations, confusion, anxiety, dizziness (vertigo), and/or abnormally fast or deep respiration (hyperventilation):
- Child (less than 55 lb (25 kg)) Observe the patient/victim and administer 0.75 mL per pound of a 25% solution (1.65 mL per kilogram of a 25% solution) of sodium thiosulfate intravenously over a period of 10 minutes.
- Adult Observe the patient/victim and administer 12.5 g of a 25% solution (50 mL of a 25% solution) of sodium thiosulfate intravenously over a period of 10 minutes.
For severe physical findings such as coma; cessation of breathing (apnea); seizures; slowness of the heart rate, usually to fewer than 60 beats per minute (bradycardia); abnormally low blood pressure (hypotension); bluish skin coloring due to abnormally low levels of oxygen in the blood (cyanosis); irregular heart beat (dysrhythmias); and/or accumulation of fluid in the lungs (pulmonary edema):- Child (less than 55 lb (25 kg)) Until sodium nitrite becomes available, break one ampule of amyl nitrite into a cloth. Out of every minute, hold the cloth containing amyl nitrite in front of the patient’s mouth for 30 seconds, and then remove it for 30 seconds, until sodium nitrite can be administered. A new ampule of amyl nitrite should be broken into a cloth every 3 minutes. Discontinue use of amyl nitrite when sodium nitrite becomes available. Administration of an entire dose (10 mL of a 3% solution) of sodium nitrite to a child can produce overwhelming lethal methemoglobinemia. Therefore, children should receive 0.15 mL per pound of body weight of sodium nitrite (0.33 mL per kg body weight of 3% sodium nitrite) over a period of 5 to 20 minutes.
- Next, administer 0.75 mL per pound body weight of 25% sodium thiosulfate (1.65 mL per kilogram body weight of 25% sodium thiosulfate) intravenously over a period of 10 minutes. If physical findings persist for 30 minutes after antidote administration, sodium nitrite and sodium thiosulfate may be readministered at half their original respective doses. However, methemoglobin levels should be monitored and not allowed to exceed 40%.
- If a child weighs more than 55 lb (25 kg), administer antidote as described for the adult (see below).
- Adult Until sodium nitrite becomes available, break one ampule of amyl nitrite into a cloth. Out of every minute, hold the cloth containing amyl nitrite in front of the patient’s mouth for 30 seconds, and then remove it for 30 seconds, until sodium nitrite can be administered. A new ampule of amyl nitrite should be broken into a cloth every 3 minutes. Discontinue use of amyl nitrite when sodium nitrite becomes available. Administer 300 mg of a 3% solution (10 mL of a 3% solution) of sodium nitrite intravenously over a period of 5 to 20 minutes.
- Next, administer 12.5 g (50 mL of a 25% solution) of sodium thiosulfate intravenously over a period of 10 minutes. If physical findings persist for 30 minutes after antidote administration, sodium nitrite and sodium thiosulfate may be readministered at half their original respective doses. However, methemoglobin levels should be monitored and not allowed to exceed 40%.
- EYE:
- Immediately remove the patient/victim from the source of exposure.
- Immediately wash eyes with large amounts of tepid water for at least 15 minutes.
- Monitor the patient/victim for signs of whole-body (systemic) effects.
- If signs of whole-body (systemic) poisoning appear, see the Inhalation section for treatment recommendations.
- Seek medical attention immediately.
- INGESTION:
- Immediately remove the patient/victim from the source of exposure.
- Establish secure large-bore IV access.
- Ensure that the patient/victim has an unobstructed airway.
- Do not induce vomiting (emesis).
- Immediately administer 100% oxygen.
- Prepare a cyanide antidote kit, for use under a physician’s direction, for symptomatic patient/victims. See the Antidote section for antidote administration procedures.
- Treat seizures with benzodiazepines.
- Seek medical attention immediately.
- INHALATION:
- Immediately remove the patient/victim from the source of exposure.
- Evaluate respiratory function and pulse.
- Ensure that the patient/victim has an unobstructed airway.
- Immediately administer 100% oxygen.
- Assist ventilation as required.
- If breathing has ceased (apnea), provide artificial respiration.
- Establish secure large-bore intravenous (IV) access.
- Prepare a cyanide antidote kit, for use under a physician’s direction, for symptomatic patients/victims. See the Antidote section for antidote administration procedures.
- Monitor for respiratory distress.
- Seek medical attention immediately.
- SKIN:
- Immediately remove the patient/victim from the source of exposure.
- See the Decontamination section for patient/victim decontamination procedures.
- Monitor the patient/victim for signs of whole-body (systemic) effects.
- If signs of whole-body (systemic) poisoning appear, see the Inhalation section for treatment recommendations.
- Seek medical attention immediately.
- MEDICAL TREATMENT: Evidence for the benefit of gastric decontamination in cases of cyanide ingestion is limited at best and should come after all other known life-saving measures have been instituted. Gastric lavage (stomach pumping) is recommended only if it can be done shortly after ingestion (generally within 1 hour), in an emergency department, and after the airway has been secured. Activated charcoal may be administered as a slurry (240 mL water/30 g charcoal). Usual dose: 25 to 100 g in adults/adolescents, 25 to 50 g in children (1 to 12 years), and 1 g/kg in infants less than 1 year old. Patient/victims who have ingested hydrogen cyanide solutions or patient/victims who have direct skin or eye contact should be observed in the Emergency Department for at least 4 to 6 hours for the development of delayed symptoms. Patient/victims with significant inhalation exposure should be monitored for the accumulation of fluid in the lungs (pulmonary edema), which may occur up to 24 to 72 hours following exposure.
- DELAYED EFFECTS OF EXPOSURE: Usually death occurs rapidly or there is prompt recovery. Survivors of severe cyanide exposures may suffer brain damage due to a direct effect of the poison (toxin) on nerve cells, or to a lack of oxygen, or possibly due to insufficient blood circulation. Examples of long-term neurological effects caused by cyanide poisoning include personality changes, memory loss, and disturbances in movement (both voluntary and involuntary movement disorders); some damage may be permanent.
- EFFECTS OF CHRONIC OR REPEATED EXPOSURE: Hydrogen cyanide has not been classified for cancer-causing (carcinogenic) effects, and no carcinogenic effects have been reported for hydrogen cyanide. No reproductive or developmental effects of hydrogen cyanide have been reported in experimental animals or humans. Chronically exposed workers may complain of headache, eye irritation, easy fatigue, chest discomfort, palpitations, loss of appetite (anorexia), and nosebleeds (epistaxis). Workers such as electroplaters and picklers, who are exposed to cyanide solutions on a daily basis, may develop a "cyanide" rash, characterized by itching and by macular, papular, and vesicular eruptions. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
- INCIDENT SITE:
- Consult with the Incident Commander regarding the agent dispersed, dissemination method, level of PPE required, location, geographic complications (if any), and the approximate number of remains.
- Coordinate responsibilities and prepare to enter the scene as part of the evaluation team along with the FBI HazMat Technician, local law enforcement evidence technician, and other relevant personnel.
- Begin tracking remains using waterproof tags.
- RECOVERY AND ON-SITE MORGUE:
- Wear PPE until all remains are deemed free of contamination.
- Establish a preliminary (holding) morgue.
- Gather evidence, and place it in a clearly labeled impervious container. Hand any evidence over to the FBI.
- Remove and tag personal effects.
- Perform a thorough external evaluation and a preliminary identification check.
- See the Decontamination section for decontamination procedures.
- Decontaminate remains before they are removed from the incident site.
See Guidelines for Mass Fatality Management During Terrorist Incidents Involving Chemical Agents, U.S. Army Soldier and Biological Chemical Command (SBCCOM), November, 2001 for detailed recommendations.
- NIOSH REL:
- Ceiling (10-min): 5 mg/m3 (4.7 ppm)
- OSHA PEL:
- TWA: 5 mg/m3 (skin) (as CN)
- ACGIH TLV:
- Ceiling: 5 mg/m3 (skin) (as CN)
- NIOSH IDLH: 25 mg/m3 (as CN)
- DOE TEEL:
- TEEL-0: 5 mg/m3
- TEEL-1: 5 mg/m3
- TEEL-2: 5 mg/m3
- TEEL-3: 60 mg/m3
- AIHA ERPG:
- ERPG-1: Not established/determined
- ERPG-2: Not established/determined
- ERPG-3: Not established/determined
| 10 min | 30 min | 60 min | 4 hr | 8 hr | |
|---|---|---|---|---|---|
| AEGL 1 (discomfort, non-disabling) - mg/m3 | 6.6 mg/m3 | 6.6 mg/m3 | 5.3 mg/m3 | 3.5 mg/m3 | 2.7 mg/m3 |
| AEGL 2 (irreversible or other serious, long-lasting effects or impaired ability to escape) - mg/m3 | 45 mg/m3 | 27 mg/m3 | 19 mg/m3 | 9.3 mg/m3 | 6.6 mg/m3 |
| AEGL 3 (life-threatening effects or death) - mg/m3 | 72 mg/m3 | 56 mg/m3 | 40 mg/m3 | 23 mg/m3 | 18 mg/m3 |
NOTE THAT VALUES ARE IN mg/m3, NOT ppm.
IMPORTANT NOTE: Interim AEGLs are established following review and consideration by the National Advisory Committee for AEGLs (NAC/AEGL) of public comments on Proposed AEGLs. Interim AEGLs are available for use by organizations while awaiting NRC/NAS peer review and publication of Final AEGLs. Changes to Interim values and Technical Support Documents may occur prior to publication of Final AEGL values. In some cases, revised Interim values may be posted on this Web site, but the revised Interim Technical Support Document for the chemical may be subject to change. (Further information is available through AEGL Process).
Decontamination (Environment and Equipment)
- ENVIRONMENT/SPILLAGE DISPOSAL: The following methods can be used to decontaminate the environment/spillage disposal:
- Do not touch or walk through the spilled agent if at all possible. However, if you must, personnel should wear the appropriate PPE during environmental decontamination. See the PPE section of this card for detailed information.
- Keep combustibles (e.g., wood, paper, and oil) away from the spilled agent. Use water spray to reduce vapors or divert vapor cloud drift. Avoid allowing water runoff to contact the spilled agent.
- Do not direct water at the spill or the source of the leak.
- Stop the leak if it is possible to do so without risk to personnel, and turn leaking containers so that gas rather than liquid escapes.
- Prevent entry into waterways, sewers, basements, or confined areas.
- Isolate the area until gas has dispersed.
- Ventilate the area.
- EQUIPMENT: Agents can seep into the crevices of equipment making it dangerous to handle. The following methods can be used to decontaminate equipment:
- Not established/determined
- Chemical Formula:KCN
- Aqueous solubility:Soluble
- Boiling Point:2957°F (1625°C)
- Density:1.52
- Flammability:Noncombustible; releases highly flammable hydrogen cyanide gas
- Flashpoint:Not established/determined
- Ionization potential:Not established/determined
- Log Kbenzene-water:Not established/determined
- Log Kow (estimated):-1.69
- Melting Point:1173°F (634°C)
- Molecular Mass:65.12
- Soluble In:Not established/determined
- Specific Gravity:1.55 at 68°F (20°C)
- Vapor Pressure:Approximately 0 mm Hg
- Volatility:Not established/determined
- Shipping Name:Potassium cyanide
- Identification Number:UN 1680 (Guide 157)
- Hazardous Class or Division:6.1
- Subsidiary Hazardous Class or Division:
- Label:Poison (Toxic)
- Placard Image:
- Cyanide of potassium
- Cyanure de potassium (French)
- Hydrocyanic acid, potassium salt
- Kalium cyanid (German)
- M-44 capsules






This is such a great resource that you are providing and you give it away for free. I love seeing blog that understand the value of providing a quality resource for freeUrinary Incontinence Treatment in Bangalore | Irregular Cycles treatment in bangalore | Pelvic Floor Dysfunction Treatment in Bangalore
BalasHapus