Canon PIXMA MX328 Driver Download For Windows
Download
|
Category
|
Windows 8.1, Windows 8 64bit
| |
Windows 8.1, Windows 8 64bit
| |
Windows 7, Windows Vista, Windows XP 32bit
| |
Windows 7, Windows Vista, Windows XP 32bit
|
Download
|
Category
|
Windows 8.1, Windows 8 64bit
| |
Windows 8.1, Windows 8 64bit
| |
Windows 7, Windows Vista, Windows XP 32bit
| |
Windows 7, Windows Vista, Windows XP 32bit
|
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Cyanide
| |
| Identifiers | |
| ChEBI | CHEBI:17514 |
| ChemSpider | 5755 |
| Jmol interactive 3D | Image |
| PubChem | 5975 |
| Properties | |
| CN− | |
| Molar mass | 26.02 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Infobox references | |

| Property | Value |
| Relative molecular mass | 27.03 |
| Boiling point (°C) | 25.70 |
| Solubility (30 °C) | Miscible with water; soluble in ethanol |
| Specific density: vapours (31 °C) | 0.937 |
| Odour threshold | 0.7 mg/m3 in air 0.17 mg/litre in water |
| Henry’s law constant (dimensionless) | 180–300b |
| Octanol/water partition coefficient (logKow) | 0.66 |
| Vapour pressure (kPa) | 35.2 at 0 °C 107.2 at 27.2 °C |
| Species | CAS number | Molecular formula | Relative molecular mass | Common synonym(s) | Boiling point (°C) | Solubility |
| Sodium cyanide | NaCN | 49.02 | Cyanide of sodium | Soluble in water, slightly soluble in alcohol | ||
| Potassium cyanide | KCN | 65.11 | Cyanide of potassium | Soluble in water, slightly soluble in alcohol | ||
| Calcium cyanide | Ca(CN)2 | 92.12 | Calcid; calsyan | Soluble in water, slightly soluble in alcohol | ||
| Copper cyanide | 54-92-3 | CuCN | 89.56 | Cupricin | Insoluble in water | |
| Potassium silver cyanide | 501-61-6 | KAg(CN)2 | 198.01 | Potassium dicyanoargentate | Soluble in water, slightly soluble in ether | |
| Sodium ferrocyanide | Na4Fe(CN)6 | 303.91 | Sodium hexacyanoferrate (II) | Soluble in water | ||
| Potassium ferrocyanide | 13943-57-3 | K4Fe(CN)6 | 368.35 | Yellow prussiate of potash | Soluble in water | |
| Potassium ferricyanide | K3Fe(CN)6 | 329.95 | Red prussiate of potash | Slowly soluble in 2.5 parts of cold water; decomposes slowly in water | ||
| Cyanogen | NCCN | 52.04 | Carbon nitrile; dicyanogen | –20.7 | Soluble in water, alcohol, and ether | |
| Cyanogen chloride | CNCl | 61.47 | Chlorine cyanide | 13.8 | Soluble in water and alcohol | |
| Acetone cyanohydrin | (CH3)2C(OH)CN | 85.10 | ACH; methyllactonitrile | 82 | Soluble in water | |
| Sodium nitroprusside | Na2[Fe(CN)5NO] | 261.97 | Sodium nitroferrocyanide; sodium nitrosyl pentacyanoferrate (III) | Soluble in 2.3 parts of water, slightly soluble in alcohol |
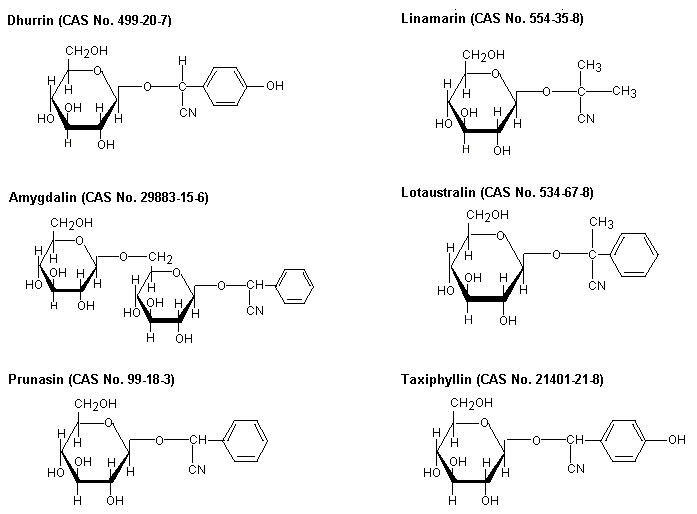
| Type of product | Cyanide concentration (in mg/kg or mg/litre) |
| Cereal grains and their products | 0.001–0.45 |
| Soy protein products | 0.07–0.3 |
| Soybean hulls | 1.24 |
| Apricot pits, wet weight | 89–2170 |
| Home-made cherry juice from pitted fruits | 5.1 |
| Home-made cherry juice containing 100% crushed pits | 23 |
| Commercial fruit juices | |
| Cherry | 4.6 |
| Apricot | 2.2 |
| Prune | 1.9 |
| Tropical foodstuffs | |
| Cassava (bitter) / dried root cortex | 2360 |
| Cassava (bitter) / leaves | 300 |
| Cassava (bitter) / whole tubers | 380 |
| Cassava (sweet) / leaves | 451 |
| Cassava (sweet) / whole tubers | 445 |
| Gari flour (Nigeria) | 10.6–22.1 |
| Sorghum / whole immature plant | 2400 |
| Bamboo / immature shoot tip | 7700 |
| Lima beans from Java (coloured) | 3000 |
| Lima beans fom Puerto Rico (black) | 2900 |
| Lima beans from Burma (white) | 2000 |
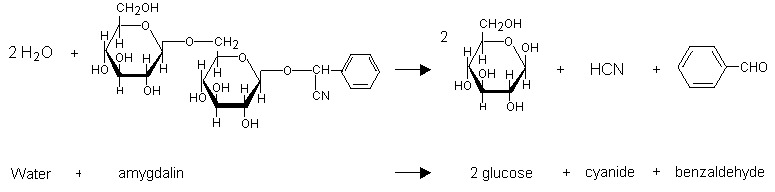
At a joint meeting of the World Federation of Associations ofClinical Toxicology and Poison Control Centres, the InternationalProgramme on Chemical Safety (IPCS), and the Commission of theHealth Organization in October 1985, the evaluation of antidotesEuropean Communities (CEC), held at the headquarters of the Worldarea for international collaboration. During 1986, the IPCS and CECused in the treatment of poisonings was identified as a prioritytherapeutic substance used to counteract the toxic action(s) of aundertook the preparatory phase of a joint project on this subject. For the purpose of the project an antidote was defined as ato treat their effects on body functions, were listed andspecified xenobiotic. Antidotes, as well as other agents used to prevent the absorption of poisons, to enhance their elimination andwere classified as: (1) those generally accepted as useful; (2)preliminarily classified according to the urgency of treatment and efficacy in practice. With respect to efficacy in practice, they those widely used and considered promising but not yet universallyused for specific purposes were considered to correspond to the WHOaccepted as useful and requiring further research concerning their efficacy and/or their indications for use; and (3) those of questionable usefulness. Additionally, certain antidotes or agents criteria for essential drugs (see Criteria for the Selection ofThese were included in volume 1 of this series.Essential Drugs, WHO Technical Report Series 722, Geneva, 1985). A methodology for the principles of evaluating antidotes and agents used in the treatment of poisonings and a proforma for preparing monographs on antidotes for specific toxins were drafted.necessary. Accordingly, several were selected for initial reviewMonographs are being prepared, using the proforma, for those antidotes and agents provisionally classified in category 1 as regards efficacy in practice. For those classified in categories 2 and 3, where there are insufficient data or controversy regarding efficacy in practice, it was agreed that further study wasToxicologists (EAPCCT; formerly known as the European Association ofand evaluation, among which were antidotes used in the treatment of poisoning by cyanide. The review and evaluation of antidotes used in the treatment of poisoning by cyanide was initiated at a joint meeting of the European Association of Poison Control Centres and Clinical Poison Control Centres), the IPCS, and the CEC, organized by thePersson, hydroxocobalamin by Professor C. Bismuth, dicobalt edetateNational Poison Information Centre of the Netherlands National Institute of Public Health and Environmental Hygiene and held at the University Hospital AZU, Utrecht, The Netherlands, 13-15 May 1987. In preparation for this meeting, documents were drafted, using the proforma, on oxygen by Dr U. Taitelman, sodium thiosulfate by Dr H. by Dr T.C. Marrs, sodium nitrite by Dr A. Hall, andNetherlands) on the clinical aspects of cyanide antidotes. The4-dimethylaminophenol by Professor M. von Clarmann. Also in preparation for the meeting, documents were drafted by Professor M. Geldmacher von Mallinckrodt on the analytical assessment of cyanide poisoning, by Dr A. van Dijk on the pharmaceutical aspects of cyanide antidotes, and by Professor A.N.P. van Heijst (formerly Director, Dutch National Poison Control Centre, Utrecht, thethe comments on the documents and of the additional materialdocuments presented by each author were discussed at the meeting and participants gave their own experience and views. Experience of industrial aspects of cyanide poisoning was presented by Dr A.C.G.M. Parren. The main meeting was followed by that of an IPCS/CEC working group, consisting of the authors of documents, the meeting rapporteur and a number of observers, at which a review was made ofmetabolic and haemoglobin abnormalities). The group concentrated onpresented at the main meeting. Based on the available material, an evaluation was made of the different approaches to treatment of cyanide poisoning depending on the type of cyanide exposure (hydrogen cyanide, either alone or with carbon monoxide, cyanide salts or cyanogenic glycosides), the state of intoxication and number of patients, the location of the patient with respect to treatment facilities, and special situations (e.g., inheritedantidotes for methaemoglobin-forming agents. Concerning theacute poisoning by cyanide, considering that there were insufficient data for evaluating approaches to treatment of chronic cyanide toxicity. Nevertheless, it was considered that a review of chronic poisoning by cyanide, particularly in relation to cyanide ingestion from food, was needed. It was agreed that traditional means of treatment of cyanide poisoning would have to be revised, and that any evaluation of approaches to treatment must also includeconsisting of Professor A.N.P. van Heijst (chairman of the meeting),analytical aspects, it was noted that there was particular difficulty in measuring the concentration of cyanide in blood if an antidote had already been administered, a problem that is being studied by a group of experts established under the auspices of the German Research Association Commission on Clinical Analytical Toxicology. A number of new cyanide antidotes in various stages of research and development were discussed. An editorial groupwas invited to the meeting but was unable to attend, prepared aDr T.J. Meredith (rapporteur), Dr J.A. Haines (IPCS, chairman of the working group) and Dr J.-C. Berger (CEC) was established in order to prepare a consolidated monograph on cyanide antidotes. Draft documents were revised by their authors. Those on methylene blue and toluidine blue were prepared by Dr Christina Alonzo (CIAT, Montevideo, Uruguay) and Dr T.C. Marrs, respectively. Subsequently Dr J.A. Vick (Food and Drug Administration, USA), who draft document on experience with the use of amyl nitrite inthe preparation and finalization of this monograph are gratefullytreating cyanide poisoning in animals. Professor C. Bismuth and Dr A. Hall drafted material on new antidotes under development for clinical trials, and Dr A.C.G.M. Parren drafted material on protective measures. The editorial group met twice in Utrecht on 22-23 October 1987 and 20-22 July 1988. Material was checked and rearranged, additional material was prepared for a number of the chapters and the overview chapter was drafted. The efforts of all who helped in acknowledged. ABBREVIATIONS ATA atmosphere absolute BE base excessOHB12 hydroxocobalaminCNS central nervous system CT computer tomography 4-DMAP 4-dimethylaminophenol EDTA ethylenediaminetetraacetic acid G6PD glucose-6-phosphate dehydrogenase Hb haemoglobin HMPS hexose monophosphate shunt INN international non-proprietary name LDLo lowest published lethal dose MLD minimal lethal dose NADH reduced nicotinamide adenine dinucleotide NADPH reduced nicotinamide adenine dinucleotide phosphateLD50 Lethal Dose 50USP United States PharmacopoeiaB12 Vitamin B12HbO2 OxyhaemoglobinAV atrioventricularSNP sodium nitroprusside VS volumetric solutionThe recognition of cyanide as a poison in bitter almonds,cherry laurel leaves, and cassava goes back to antiquity. Anrefers to the "penalty of the peach," and Dioscorides in the firstinscription on an Egyptian papyrus in the Louvre Museum, Paris,The first description of cyanide poisoning was by Wepfer incentury A.D. was aware of the poisonous properties of bitter almonds (Sykes, 1981).Ireland caused by drinking cherry laurel water, used as a flavouring1679 and dealt with the effects of the administration of extract of bitter almonds (Sykes, 1981). Two fatal cases of poisoning inpoison; given orally, into the rectum, or by injection, it rapidlyagent in cooking and to dilute brandy, led to the experiments of Madden (1731). He showed that cherry laurel water contains a killed dogs. It was not until 1786 that isolation of pure hydrogenSchrader (1802). The introduction of cyanide as a medicament tocyanide (HCN) from the dye Prussian blue was achieved by Scheele (1786). The mechanism of toxicity of cyanide was explored by Fontana (1795). Cyanide was obtained from bitter almonds by treat coughs and lung diseases was suggested by Magendie (1817).Hoppe-Seyler (1876) reported that cyanide inhibits tissue oxidationIndeed, it was not until 1948 that cherry laurel water was removed from the British Pharmacopoeia! Attempts to antagonize the toxic effects of cyanide were reported by Blake (1839 and 1840). reactions. Antagonism between amyl nitrite and prussic acid was mentionedChen et al. (1933, 1934). They suggested using a combination ofby Pedigo (1888), and, as early as 1894, cobalt compounds were advocated by Antal (1894) as cyanide antagonists. Sodium nitrite was used as an antidote in experimental cyanide poisoning by Mladoveanu & Gheorghiu (1929). A biochemical mechanism for cyanide antagonism was described bytoxicological reasoning. This combination of antidotes has stoodamyl nitrite, sodium nitrite and sodium thiosulfate, the latter compound serving as a sulfur donor for rhodanese (thiosulfate sulfur transferase). Rhodanese accelerates cyanide detoxification by forming the metabolite thiocyanate. This represented the development of one of the first antidotes based on scientificB12a) combined with cyanide to form cyanocobalamin (vitaminthe test of time, and still represents one of the most efficacious antidotal combinations for the treatment of cyanide intoxication. Interest in cobalt compounds was renewed by Mushett et al. (1952), who demonstrated in 1952 that hydroxocobalamin (vitaminB12).Paulet (1960) subsequently reported that cobalt EDTA was moreeffective as a cyanide antidote than the classic nitrite-thiosulfatecombination.Hydrogen cyanide is used in the fumigation of ships, largebuildings, flour mills, private dwellings, freight cars, andbound to a carrier, commonly diatomaceous earth, and blended with anaeroplanes that have been infested by rodents or insects. It is odorous or irritating product as a warning marker.Halogenated cyanides (chloro-, bromo- and iodocyanide) inCyanide salts are utilized in metal cleaning, hardening, ore-extracting processes, and electroplating. contact with water produce the non-toxic cyanic acid. As a resultis used as a solvent and is less toxic (LD50 = 120 mg/kg) thanof contact with strong acids, hydrogen cyanide is liberated. Nitriles are cyano-derivatives of organic compounds. Acetonitrilehydrogen cyanide (LD50= 0.5 mg/kg), but often contains toxicadmixtures due to metabolism to inorganic cyanide. While aliphatic nitrilesmetabolise to inorganic cyanide, the aromatic nitrile bond is stablein vivo. Acrylonitrile is the raw material used for themanufacture of plastics and synthetic fibres. Contact with skincauses bullae formation. Pyrolysis generates hydrogen cyanide.Acrylonitrile and propionitrile are less toxic (LD50 = 35 mg/kg)than butyronitrile (LD50 = 10 mg/kg). Trichloroacetonitrile(LD50 = 200 mg/kg) is used as an insecticide. The aromaticnitriles, bromoxynil (LD50= 190 mg/kg) and ioxynil (LD50= 110mg/kg), are used as herbicides.Cyanamide, cyanoacetic acid, ferricyanide and ferrocyanide donot release cyanide. They are therefore less toxic (LD50=1000-2000 mg/kg) than the cyanogenic compounds above, though theymay cause toxicity by other means, e.g. cyanide in combination withalcohol.Fires and automobile pollution-control devices withmalfunctioning catalytic converters (Voorhoeve et al., 1975)generate cyanide. Natural substances, such as wool, silk, horsehair, and tobacco, as well as modern synthetic materials, such aspolyurethane and polyacrylonitriles, release cyanide duringHarland, 1982; Clark et al., 1983; Alarie, 1985; Lowry et al., 1985)combustion (Levine et al., 1978; Birky et al., 1979; Anderson & (Table 1).Table 1. Hydrogen cyanide generated by pyrolysisµg HCN perMaterial g materialpaper 1100wool 6300cotton 130 nylon 780polyurethane foam 1200From: Montgomery et al. (1975)Cyanide is found in foodstuffs such as cabbage, spinach, andalmonds, and as amygdalin in apple pips, peach, plum, cherry, andcompletely harmless as long as it is relatively dry. However, thealmond kernels. In the kernels themselves, amygdalin seems to beC20H27NO11 + 2H2O --> 2C6H12O6 + C6H5CHO + HCNseeds contain an enzyme that is capable of catalysing the following hydrolytic reaction when the seeds are crushed and moistened:amygdalin glucose benzaldehyde hydrogencyanideNatural oil of bitter almonds contains 4% HCN. American whiteThe reaction is slow in acid but rapid in alkaline solution. lima beans contain 10 mg cyanide/100 g bean. The dried root ofto be about 9 mg and that in wild apricot seeds more than 200 mg.cassava (tapioca) may contain 245 mg cyanide/100 g root. The cyanide content in 100 g of cultivated apricot seeds has been foundCyanide is also formed during nitroprusside therapy, especiallywhen it is prolonged, because tachyphylaxis sometimes requires the(Smith & Kruszyna, 1974; MacRae & Owen, 1974; Piper, 1975; Atkins,use of higher doses than the recommended maximum of 10 µg/kg per minantihypertensive agents and they saw wide use because they were very1977; Anon, 1978). Cyanide metabolises to thiocyanate. Thiocyanates were used some years ago asled to their disfavour.effective. However, a variety of subacute toxic effects, including anorexia, fatigue, and gastrointestinal tract and CNS disturbances,effect could not be demonstrated in either retrospective orLaetrile, amygdalin derived from apricot kernels, has been used as an anticancer agent, but it is now obsolete because a therapeutic prospective studies. Laetrile has caused fatal cyanide poisoning (Sadoff et al., 1978).It is generally accepted that inhalation of approximately 50 ml(at 1.85 mmol/l) of hydrogen cyanide gas is fatal within minutes.accidental than suicidal. Thus accidental cyanide poisoning mayPoisoning from hydrogen cyanide is more frequentlycourse of their work (Chen et al., 1944). In fires, a combinationoccur in fumigators and chemists who use hydrogen cyanide during theSuicidal ingestion of cyanide salts most commonly occurs inof HCN and carbon monoxide (CO) toxicity, as a result of inhalation of combustion products, may cause fatalities.et al., 1982). However, death may be delayed for several hourspersonnel with occupational access to cyanide. The ingestion of as little as 250 mg of an inorganic cyanide salt may be fatal (Peters following the ingestion of cyanide on a full stomach; a first-pass effect in the liver may also delay the onset of toxicity (Naughton, 1974).Chronic low-dose neurotoxicity have been suggested byepidemiological studies of populations ingesting naturally occurringplant glycosides (Blanc et al, 1985). These glycosides are presenta major tropical foodstuff (Conn, 1973; Cook & Coursey, 1981;in a wide variety of plant species, most notably the cassava plant, Ministry of Health, Mozambique, 1984). Cassava has been associatedcyanide and a low sulfur intake from diets dominated bywith tropical ataxic neuropathy (Cook & Coursey, 1981). Epidemic spastic paraparesis has been associated with a combination of a high insufficiently processed cassava and lacking protein supplementaryal., 1983). Long-term cyanide intoxication has been shown to befood (Rosling, 1989). A neurotoxicological role for cyanide has also been suggested in tobacco-associated amblyopia (Grant, 1980) and in amygdalin-associated peripheral neuropathy (Kalyanaraman et associated both with thyroid gland enlargement and dysfunction in case reports and in cohort studies of individuals exposed occupationally (Blanc et al., 1985), through dietary intake (Cook & Coursey, 1981), and experimentally (El Ghawabi et al., 1975).Cyanide has a special affinity for the ferric ions that occurin cytochrome oxidase, the terminal oxidative respiratory enzyme inutilization of oxygen. When cytochrome oxidase is inhibited bymitochondria. This enzyme is an essential catalyst for tissue cyanide, histotoxic anoxia occurs as aerobic metabolism becomesrelease of biogenic amines may play a role by causing cardiacinhibited. In massive cyanide poisoning, the mechanism of toxicity is more complex. It is possible that autonomic shock from thedecrease in cardiac output. This theory is supported by the sharpfailure (Burrows & Way, 1976). Cyanide could cause both pulmonary arteriolar and/or coronary arterial vasoconstriction, which would result, either directly or indirectly, in pump failure and aobservation that phenoxybenzamine, an alpha-adrenergic blockingincrease in central venous pressure that was observed by Vick & Froelich (1985) at a time when the arterial blood pressure fell after the intravenous administration of sodium cyanide to dogs. Thenitrite, a potent arteriolar vasodilating agent, resulted in thedrug, partially prevented these early changes (Vick & Froelich, 1985) supports the concept of an early shock-like state not related to inhibition of the cytochrome oxidase system. Inhalation of amyl survival of dogs in these experimental circumstances. This could have been due to reversal of early cyanide-induced vasoconstriction with restoration of normal cardiac function (Vick & Froelich, 1985).The smell of bitter almonds in expired air is an important signin cyanide poisoning. However, many people are unable to perceiveincidence of "non-smellers" is reported to be 18% among males and 5%the odour of hydrocyanic acid (Kalmus & Hubbard, 1960). The among females (Kirk & Stenhouse, 1953; Fukumoto et al., 1957).experienced. A blood-stained aspirate may be observed if gastricImmediately after swallowing cyanide, very early symptoms, such as irritation of the tongue and mucous membranes, may be lavage is performed. Early symptoms and signs that occur aftercyanosis, hypotension, bradycardia, and sinus or AV nodalinhalation of HCN or the ingestion of cyanide salts include anxiety, headache, vertigo, confusion, and hyperpnoea, followed by dyspnoea, arrythmias. In the secondary stage of poisoning, impaired consciousness,hypotension, complex arrythmias, cardiovascular collapse, pulmonarycoma and convulsions occur and the skin becomes cold, clammy, and moist. The pulse becomes weaker and more rapid. Opisthotonos and trismus may be observed. Late signs of cyanide toxicity include oedema, and death.the high concentration of oxyhaemoglobin in the venous return, but,It should be emphasized that the bright-red coloration of the skin or absence of cyanosis mentioned in textbooks (Gosselin et al., 1984; Goldfrank et al., 1984) is seldom described in case reports of cyanide poisonings. Theoretically this sign could be explained bydifferent mechanisms: (1) an intracellular metabolic process thatespecially in massive poisoning, cardiovascular collapse will prevent this from occurring. Sometimes, cyanosis can be observed initially, while later the patient may become bright pink (Hilmann et al., 1974). The pathogenesis of pulmonary oedema could be due to severalpoisoning and it has been shown that cyanide significantly increasescould injure the alveolar and capillary epithelium directly, producing a capillary leak syndrome; (2) neurogenic pulmonary oedema or, (3) most likely, a direct effect on the myocardium leading to left ventricular failure and increased pulmonary venous pressure. The brain is obviously the key organ involved in cyanide brain lactate and decreases brain ATP concentrations (Olsen & Klein, 1947).Since oxidative phosphorylation is blocked, the rate ofglycolysis is markedly increased, which in turn leads to lacticacidosis. The degree of lactic acidosis can be correlated with theseverity of cyanide poisoning (Trapp, 1970; Naughton, 1974).A reversible toxic effect occurs on the pancreatic beta-cells,which may occasionally give rise to an erroneous diagnosis of hyperglycaemic diabetic coma.Before intravenous treatment with antidotes is commenced, it isnecessary to collect a heparinized (not fluoride) blood sample forcollected after treatment are totally unreliable. A quantitativedetermination of cyanide concentration. Results from samples test employing a detector tube (see chapter 10) can be used if thepoisoning can be evaluated. Therapeutic measures after antidotaldiagnosis is in doubt. The blood can also be used for a quantitative test (see chapter 10), so that the severity of treatment should be based on the clinical condition of the patientto 0.005-0.04 mg/l have been recorded in healthy non-smokers, andrather than on blood cyanide concentrations (Berlin, 1971; Vogel et al., 1981; Peters et al., 1982). Since blood concentrations of up 0.01-0.09 mg/l in smokers, only concentrations above these values(plasma), 6.0 µg/l (erythrocytes); smokers 8.6 µg/l (whole blood),were previously considered to be toxic (Vogel et al., 1981; Peters et al., 1982). Lundquist et al., (1985) reported even lower concentration: non-smokers 3.4 µg/l (whole blood), 0.5 µg/l 0.8 µg/l (plasma), 17.7 µg/l (erythrocytes).bound. A plasma-to-blood ratio as high as 1:10 has been reportedFatal cyanide poisoning has been reported with whole blood concentrations of >3 mg/l and severe poisoning with 2 mg/l (Graham et al., 1977). However, when cyanide enters the bloodstream, up to 98% quickly enters the red blood cells where it becomes tightly1976). However, a serious drawback to the use of plasma cyanideand, as a consequence, the whole blood cyanide concentration may not accurately reflect tissue concentrations of cyanide. Plasma levels of cyanide may be of greater significance because severe toxicity occurs in the presence of only modest concentrations (Vesey et al., determinations in the assessment of poisoning is the pronounced instability of cyanide in plasma (Lundquist et al., 1985).The major pathway of endogenous detoxification is conversion,by means of thiosulfate, to thiocyanate. Minor routes of eliminationare excretion of hydrogen cyanide through the lungs and binding.Metabolic Detoxification of Cyanide;V02ANnew.BMPto cysteine or hydroxocobalamin. The detoxification of cyanide occurs slowly at the rate ofenzyme is needed to catalyse the transfer of a sulfur atom0.017 mg/kg per min (McNamara, 1976). A sulfurtransferase from the donor thiosulfate to cyanide. The classical theorydoubt because thiosulfate penetrates lipid membranes slowlyindicating that mitochondrial thiosulfate sulfurtransferase is the most important enzyme in this reaction is now in and would, therefore, not be readily available as aprimary cyanide detoxification buffer operating in normal metabolismsource of sulfur in cyanide poisoning. The modern concept assumes a greater role for the serum albumin-sulfane complex, which is the (Sylvester et al., 1983). A further enzyme, beta-mercaptopyruvate sulfurtransferase, also converts cyanide to thiocyanate (Vesey et al., 1974). This enzyme is found in the erythrocytes, but in human cells its activity is low.The detoxification product of cyanide, thiocyanate, is excretedin the urine. Thiocyanate concentrations are normally betweenplasma half-life of thiocyanate in patients with normal renal1-4 mg/l in the plasma of non-smokers and 3-12 mg/l in smokers. Thetherefore at increased risk of toxicity (Schulz et al., 1978).function is 4 h (Blaschle & Melmon, 1980), but in those with renal insufficiency it is markedly prolonged and these patients aremuscle spasm, nausea, disorientation, psychosis, hyper-reflexia, andThiocyanate levels exceeding 100 mg/l are thought to be associated with toxicity. Thiocyanate toxicity is characterized by weakness,1931; Garvin, 1939; Russel & Stahl, 1942; Kessler & Hines, 1948;stupor (Smith, 1973; Michenfelder & Tinker, 1977). Lethal poisoning at concentrations greater than 180 mg/l has been reported (Healy, Domalski et al., 1953). Haemodialysis is recommended as anDialysance values of 82.8 ml/min ( in vivo) and 102.3 ml/mineffective means of removing thiocyanate (Marbury et al., 1982).( in vitro) have been recorded (Pahl & Vaziri, 1982). Little isknown about the protein-binding characteristics of thiocyanate, andhaemoperfusion may be more effective than haemodialysis.Accidental exposure to cyanide, as either hydrogen cyanide orcyanide salts, will occur primarily in the occupational context, andappropriate preventive and protective measures need to be takenwherever cyanides are manufactured or used. Many industrial accidentsoccur as a result of mixing cyanide salts and acids, and care should be taken when both are present on industrial premises.The public may be affected in the case of a major industrialAs hydrogen cyanide may be generated during combustion of organic substances, fire fighters may also be exposed occupationally.cyanides are used to have contingency plans that will enable them toemergency, or of a transport accident, involving the release of cyanides. It is essential for local authorities in areas whereindustrial hygiene are essential for the prevention of cyaniderespond effectively. Adequate hospital facilities for treatment of casualties must be available. Proper maintenance of plant, good operating practice, andpeople in zones where cyanide could be released accidentally. Therepoisoning. Areas in the workplace where cyanides are used and containers for storage and transport of cyanide should be clearly marked. Work schedules should ensure that there are at least twotoxic materials, such as dirty and clean locker facilities andshould be showers and first-aid kits in these areas. Personnel without proper training should not be allowed in the plant. Normal industrial and laboratory hygiene measures for personnel handlingcyanides, should receive instruction on the dangers of cyanides andshowers, should be provided. Eating, drinking, and smoking should not be allowed in the work area where cyanides are used but in places specially reserved for these purposes. Each employee working at a plant or laboratory that handlessymptoms and signs of cyanide poisoning and how to achieve safebe trained in appropriate first-aid measures, as should emergency-service personnel. They should be aware of the hazards and informed about the possible routes of exposure (inhalation, skin absorption, ingestion). Training should involve recognition of theregular instruction sessions covering procedures for handlingremoval of victims from the source of intoxication. Personnel should also be able to guide a rescue or fire-fighting team to a trapped intoxicated person. Rescue personnel should be able to put on protective clothing quickly in an emergency. There should beand decontamination of exposed skin and eyes. It should be realizedcyanides and for rescue in case of accidents, as well as random alarm exercises. First-aid training should include the essential measures to be taken before medical help arrives, which may need to be undertaken at the same time as removal of contaminated clothingin plants where the gas is used or may be generated. Warningthat further uptake of cyanide into the blood may occur after showering because of continued skin absorption. Each plant handling cyanide should have its own medical staff trained in the emergency treatment of cyanide poisonings. The atmospheric concentrations of hydrogen cyanide should be monitoredFilter respirators should be carried at all times by employeesdevices are available for this purpose and should be installed. In certain circumstances in which cyanide is used, it is possible to add a warning gas, e.g., cyanogen chloride and chloropicrin have been added to hydrogen cyanide used as a fumigant (Cousineau & Legg, 1935; Polson & Tattersall, 1969). working in zones where hydrogen cyanide may be released. At highworker should be aware of the emergency procedures to be followedhydrogen cyanide concentrations, absorption occurs through the skin and impermeable butyl rubber protective clothing is required. Oxygen breathing apparatus may be needed. In the case of an accident involving hydrogen cyanide there should be both an acoustic and a visual alarm for the plant, which may be activated by workers in zones where the gas is used. Eachhave the authority and training to perform the special resuscitationand the protective clothing and equipment to be used. If a large number of victims is involved or if there is a danger to the public, local authorities need to be warned, so that contingency plans are put into effect and hospitals alerted. For accidents at plants in remote areas where a qualified physician is not readily available and there are no hospital intensive care facilities, attending paramedical personnel should measures involved in treating cyanide poisonings, including rapid endotracheal intubation and techniques for obtaining intravenous access.Although effective antidotes are available, general supportivemeasures should not be ignored and may be life-saving.experience of 104 industrial poisoning cases, the use of specificAccording to Jacobs (1984), who reported his personaldeep coma, wide non-reactive pupils, and respiratory insufficiencyantidotes was indicated only in cases of severe intoxication withmoderately severe poisoning, who had suffered only a brief period ofin combination with circulatory insufficiency. In patients with unconsciousness, convulsions, vomiting, and cyanosis, therapycases of mild intoxication with dizziness, nausea, and drowsiness,consisted of intensive care and intravenous sodium thiosulfate. In rest and oxygen alone were used. Peden et al. (1986) described nine patients poisoned byfrom the area where they had been working. The arterial whole-bloodhydrogen cyanide released by a leak from a valve. Three of them were briefly unconscious but recovered rapidly after being moved cyanide concentrations on admission were 3.5, 3.1 and 2.8 mg/l,two were transiently unconscious, and in these cases the cyaniderespectively. The cyanide concentrations in the other cases ranged between 2.6 and 0.93 mg/l. All recovered with supportive therapy alone. Between 1970 and 1984, three other men were treated similarly;comatose patients with potentially lethal blood concentrations onconcentrations 30 min after exposure were 7.7 and 4.7 mg/l. The concentration in the other patient was 1.6 mg/l. All three patients recovered without the use of cyanide antidotes. Small numbers of admission, and who recovered without cyanide antidotes, have beenA patient exposed to hydrogen cyanide who reaches hospitalreported by Graham et al. (1977), Edwards & Thomas (1978), and Vogel et al. (1981). Even if a patient is unconscious, an antidote does not necessarily have to be administered immediately unless vital signs deteriorate. fully conscious is only likely to require observation and reassurance. 1.9.2.1 OxygenIt is difficult to understand how oxygen has a favourableeffect in cyanide poisoning, because inhibition of cytochromeregarded as an important first-aid measure in cyanide poisoning, andoxidase is non-competitive. However, oxygen has always been there is now experimental evidence that oxygen has specificinhibition by cyanide (Takano et al., 1980). Nevertheless, thereantidotal activity. Oxygen accelerates the reactivation of cytochrome oxidase and protects against cytochrome oxidasesuffering from combined carbon monoxide and cyanide poisoning, sinceare other possible modes of action and those that are clinically important have yet to be determined. Hyperbaric oxygen is recommended for smoke inhalation victimsThe major route of cyanide detoxification in the body isthese two agents are synergistically toxic. The use of hyperbaric oxygen in pure cyanide poisoning remains controversial. 1.9.2.2 Sodium thiosulfate conversion to thiocyanate by rhodanese, although otherCyanide poisoning is an intramitochondrial process and ansulfurtransferases, such as beta-mercaptopyruvate sulfurtransferase, may also be involved. This reaction requires a source of sulfane sulfur, but endogenous supplies of this substance are limited. intravenous supply of sulfur will only penetrate mitochondriafor example in cases of smoke inhalation. Sodium thiosulfate isslowly. While sodium thiosulfate may be sufficient alone in mild to moderately severe cases, it should be administered with other antidotes in cases of severe poisoning. It is also the antidote of choice when the diagnosis of cyanide intoxication is not certain,for many years as a simple first-aid measure that generatesassumed to be intrinsically nontoxic but the detoxification product formed from cyanide, thiocyanate, may cause toxicity in patients with renal insufficiency (see section 1.7). 1.9.2.3 Amyl nitrite The administration of amyl nitrite by inhalation has been usedrecent studies suggest that methaemoglobin formation plays only amethaemoglobin and which can be employed by lay personnel. Its use was abandoned because the methaemoglobin concentration obtained with amyl nitrite is no more than 7% and it is thought that at least 15% is required to bind a potentially lethal dose of cyanide. However, small role in the therapeutic effect of amyl nitrite, andNitrites generate methaemoglobin, which combines with cyanidevasodilatation may be the most important mechanism of antidotal action. Artificial respiration with amyl nitrite ampoules broken into an Ambu bag proved to be life-saving in dogs severely poisoned with cyanide. Amyl nitrite may therefore be reintroduced as a first-aid measure. 1.9.2.4 Sodium nitrite to form the nontoxic substance cyanmethaemoglobin. Methaemoglobinhaemoglobin should be monitored to ensure aerobic metabolism of thedoes not have a higher affinity for cyanide than does cytochrome oxidase, but there is a much larger potential source of methaemoglobin than there is of cytochrome oxidase. The efficacy of methaemoglobin is therefore primarily the result of mass action. A drawback of methaemoglobin generation is the resultant impairment of oxygen transport to cells and, ideally, the total amount of freelikely to be less because only low plasma concentrations arecells. Methaemoglobin can be measured very quickly, but this in itself will not provide an accurate guide to the amount of haemoglobin available for oxygen transport because the cyanmethaemoglobin concentration is not taken into account. Individuals deficient in glucose-6-phosphate dehydrogenase (G6PD) are at great risk from sodium nitrite therapy because of the likelihood of severe haemolysis, but the risk from amyl nitrite isassociated with methaemoglobin formation, as described above forachieved. Excess methaemoglobinaemia may be corrected with either methylene or toluidine blue (see Chapter 9) or, preferably, where feasible, by exchange transfusion. 1.9.2.5 4-Dimethylaminophenol (4-DMAP) 4-DMAP generates a methaemoglobin concentration of 30-50% within a few minutes (Weger, 1968) and, theoretically, it should therefore be valuable as a first-aid measure. However, the problems nitrites, apply to 4-DMAP to an even greater extent. Furthermore,1.9.2.6 Hydroxocobalamin (vitamin Bl2a)it has very poor dose-response curve reproducibility. Haemolysis as a result of 4-DMAP therapy has been observed in overdose as well as following a correct therapeutic dose. Treatment with 4-DMAP is contraindicated in patients with G6PD deficiency. Excess methaemoglobinaemia may be corrected with either methylene or toluidine blue (see section 1.9.2.8).This antidote binds cyanide strongly to form cyanocobalamin(vitamin B12) and, compared to nitrite and 4-DMAP therapy, it hasthe great advantage of not interfering with tissue oxygenation. Thedisadvantage of hydroxocobalamin as a cyanide antidote is the largecyanide (corresponding to 65 mg KCN) needs 1406 mg hydroxocobalamin.dose required for it to be effective. Detoxification of 1 mmol In most countries it is only commercially available in formulationsthat has to be reconstituted with 80 ml of a 10% sodium thiosulfateof 1-2 mg per ampoule. In some countries, e.g., France, a formulation is available that contains 4 g hydroxocobalamin powderreactions and acne. Some authors have reported a reduced antidotalsolution prior to use and administered intravenously in a minimum of 220 ml of 5% dextrose. Recorded side effects are anaphylactoidHistological changes in the liver, myocardium, and kidney apparentlyeffect as a result of mixing hydroxocobalamin and sodium thiosulfate in the same solution (Evans, 1964; Friedberg & Shukla, 1975). induced by hydroxocobalamin have been reported in animal1.9.2.7 Dicobalt edetateexperiments (Hoebel et al., 1980), but their relevance to man has not yet been established. Transient pink discoloration of mucous membranes and urine is an unimportant and nontoxic side-effect.definitely present. This is a strict criterion, because as a resultThis agent has been shown to be effective in the treatment of cyanide poisoning in man, and in the United Kingdom it is the current treatment of choice provided that cyanide toxicity is of the manufacturing process some free cobalt ions are alwaysthat glucose protects against cobalt toxicity and it is recommendedpresent in solutions of dicobalt edetate. Cobalt ions are toxic and the use of dicobalt edetate, in the absence of cyanide, will lead to serious cobalt toxicity. There is evidence from animal experiments that this be given at the same time as dicobalt edetate. Seriousconcentrations in the presence of cyanide is difficult.adverse effects recorded include vomiting, urticaria, anaphylactic shock, hypotension, and ventricular arrhythmias (Hilmann et al., 1974; Naughton, 1974). 1.9.2.8 Antidotes to methaemoglobin-forming agents Accurate determination of methaemoglobin and free haemoglobin Nevertheless, excess methaemoglobinaemia does undoubtedly occur on occasions following the use of nitrites and 4-DMAP. Excess methaemoglobin concentrations may be reduced by methylene or toluidine blue. However, regeneration of haemoglobin will release cyanide back into the circulation, leading to a recurrence of toxicity.The management of cyanide poisoning is determined by (i) thenature of exposure, i.e. hydrogen cyanide (with or without carbonmonoxide), cyanide salts, aliphatic nitriles, cyanogenic glycosides;(ii) the severity of poisoning; (iii) the number of patientsinvolved; (iv) the proximity of hospital facilities;